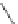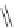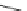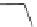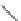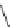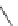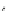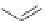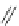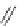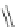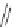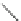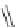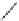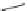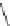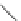Chemistry Reference
In-Depth Information
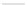
Cl
FVP
1100 ºC
FVP
1100 ºC
20%
Cl
16%
Cl
84
Cl
85
86
Scheme 24 Synthesis of benzocorannulene 85 [
54
,
115
,
116
]
1)
O
Ar
Ar
O
O
Ar
Ar
Ar
Ar
10
RhCl(PPh
3
)
3
KOH, (CH
2
OH)
2
110 °C, 60 h
p-xylene
9
75%
2)
87
palladacycle
DBU, DMF
150 °C
57%
40%
FVP, 1000 °C
38%
Ar =
R
R
O
O
P
Pd
OO
Pd
Br
P
R
R
R = o-tol
Palladacycle
88
Scheme 25 Synthesis of dibenzo[
a
,
g
]corannulene (88)[
16
,
117
,
118
]
n-C
8
H
17
Pd(PCy
3
)
2
Cl
2
DBU
N
Br
Br
O
O
DMAc, 145 °C
36%
90
89
91
Br
Br
FVP, 1100 ºC
9%
93
Cl
92
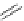
Scheme 26 Synthesis of buckybowls 90 and 93 [
119
,
121
]
cyclization and an aryl radical is generated by loss of a chlorine atom. Benzo-
corannulene 85 has also been prepared in the solution phase. Refluxing with
Fe
2
(CO)
9
in benzene, 31 was converted quantitatively to benzocorannulene 85 by
cleavage of the 'endoxode' bridge [
54
].