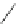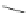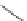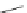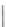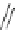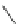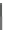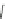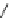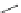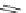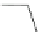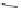Chemistry Reference
In-Depth Information
RO
Br
RO
RO
Cl
Br
OR
OR
Cl
OR
DMF
SOCl
2
Br
2
RO
Br
RO
RO
Cl
Br
Cl
OR
OR
OR
RO
RO
RO
Br
Cl
48
-OR
59
-Br
59
-Cl
R=Me(87%)
heptyl (42%)
R=Me(78%)
heptyl (62%)
Scheme 17 Synthesis of deca-heterosubstituted corannulenes 59 [
93
]
butylphenyl)corannulene and decakis(4-chlorophenyl)corannulene to be obtained
in ca. 23% and 54% yields, respectively.
Reaction of decachlorocorannulene (52) with a variety of S-nucleophiles is a
general protocol for synthesis of per(1-thio)-substituted corannulenes 58
(Scheme
16
). Scott et al. efficiently prepared various decakis(1-alkylthio)
corannulenes 58 by treating 52 with the corresponding sodium thiolates at room
temperature [
100
]. Similarly, decakis(phenylthio)corannulene (58a) was obtained
in 50% yield [
75
]. For the synthesis of 58b and 58c, the thiol species of the
corresponding paraffinic components were ionized in situ by using DBU for the
reaction with decachlorocorannulene (52). Corannulene derivatives 58b and 58c
were found to form a liquid crystalline assembly [
101
].
Decasubstituted corannulenes can also be generated by functionalization of
pentasubstituted corannulenes. The alkoxy groups on 48 increase electron density
on corannulene to offer opportunities for halogenation to produce decahetero-
substituted corannulenes 59 with two different substituents under mild conditions
(Scheme
17
). The treatment of pentaalkoxycorannulenes 48 with excess Br
2
or
excess thionyl chloride afforded 59-Br and 59-Cl, respectively [
93
].
2.2.2 Structures and Properties of Highly Substituted Corannulenes
The structures and properties of highly substituted corannulenes strongly depend on
the number and type of substituents. In the subclass of alkyl-substituted
corannulenes, decamethylcorannulene (54) is much shallower than pentamethyl-
corannulene 45-Me (Table
1
). The calculated
G
{
inv
and bowl depth for 54 are only
ʔ
2.2 kcal/mol and 0.58
,
respectively [
62
]. Similarly, decakis(phenylthio)-
Å
corannulene (58a, 0.49
)[
75
] is flatter than pentakis(phenylthio)corannulene 48
Å
(XR
¼
SPh), which contains two different molecules to have the bowl depths 0.87
Å
and 0.91
depending on orientations of the thiophenyl substituents (Siegel et al.,
unpublished results). Due to the fact that five phenylthio substituents are each
oriented above and below the rim in 58a, its inversion dynamics are complex.
Moreover, the addition of bulky groups to the corannulene core also flattens the
bowl. For example,
Å
the bowl depth of 1,3,5,7,9-penta(
tert
-butyl)corannulene