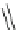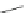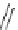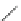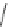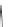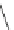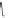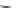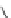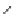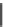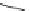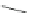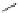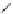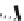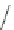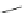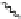Chemistry Reference
In-Depth Information
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
SO
2
Cl
2
AlCl
3
,S
2
Cl
2
60%
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
1
Cl
Cl
Cl
Cl
NiCl
2
(dppp)
AlMe
3
,DME
52
53
R
-
SnMe
3
t
-BuO
K,
DME
R=C
Pd(OAc)
2
IPr HCl
30%
C
n-
Bu
n-
Pr
n-
Pr
R
R
R
R
R
R
+
R
R
R
R
54
R
R
R
R
R
R
R
R
55
56
10%
18%
Ar
Ar
Ar
Ar
Ar
Pd(OAC)
2
o
-chloranil
B
O
B
O
+
Ar
Ar
B
repeat 3
-
4cycles
Ar = Ph (6%)
4-
t
-Bu-C
6
H
4
(23%)
4-Cl-C
6
H
4
(54%)
Ar
O
Ar
Ar
Ar
1
Ar
Ar
57
Scheme 15 Synthesis of decasubstituted corannulene derivatives [
34
,
49
,
62
,
71
,
74
,
98
,
99
]
RS
SR
58a
(R = Ph, 50%)
RS
SR
N
n-
C
10
H
21
58b
(R =
, 37%)
NaSR
O
RS
52
SR
or RSH, DBU
O
n-
C
12
H
25
O
n-
C
12
H
25
RS
SR
RS
SR
58c
(R =
, 7%)
N
58
O
n-
C
12
H
25
R=alkyl,aryl
O
Scheme 16 Synthesis of decakis(1-thio)corannulenes 58 [
75
,
100
,
101
]
observed that the arylation was not stopped at decaphenylcorannulene 57 (Ar
Ph)
when phenylboroxin was employed, whereas (4-
tert
-butylphenyl)boroxin and
(4-chlorophenyl)boroxin prevent
¼
the overarylation and allow decakis(4-
tert
-