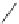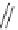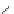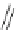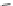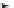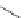Chemistry Reference
In-Depth Information
R
2
R
1
R
2
R
1
R
2
R
1
R
2
R
1
O
O
51
45
R
2
R
1
O
O
51
-pL [R
2
= (CH
2
)
3
O
45
-PrOH [R
1
= (CH
2
)
3
OH]
]
O
O
H
n
N
NMe
2
O
Br
Br
Ph
pyridine
O
Cu(I)
45
-PrE [R
1
= (CH
2
)
3
O
51
-pS [R
2
= (CH
2
)
3
O
]
]
Br
Ph
n
O
Br
Scheme 14 Synthesis of corannulene-core polymers 51 [
97
]
The initiators 45-PrOH and 45-PrE were then used to polymerize cyclic ester and
styrenic monomers to give poly(lactide)s 51-pL and polystyrene 51-pS, respec-
tively. Both polymerizations were observed under reasonable control over the
molecular weight and chemical nature of the arms. Preliminary results demon-
strated that these polymers are capable of forming a host-guest complex with C
60
.
Balister conditions, using sulfonyl chloride, aluminum chloride, and sulfur
dichloride, produced a sparingly soluble decachlorocorannulene 52 [
34
,
49
],
which should be generated via the intermediate 53 by loss of two chlorine mole-
cules [
98
] (Scheme
15
). In contrast to these confirmed perchlorinations of
corannulene, Huang et al. reported a compound, isolated from electrical discharge
in liquid chloroform, which they asserted to be 52 [
99
]; however, that product
differs greatly in solubility, does not show the same NMR as authentic 52, and has
never been used to form authenticated decasubstituted products of corannulene.
Therefore, the structure of the compound synthesized by the electrical discharge
protocol is highly unlikely to be 52 and a proper structure determination is
needed [
71
].
Several corannulene derivatives were obtained by metal-catalyzed coupling
reaction of decachlorocorannulene (52). Decamethylcorannulene 54 was synthe-
sized in 30% yield by methylations of 52 in the presence of NiCl
2
(dppp) and
trimethylaluminum [
62
]. Palladium-catalyzed cross-coupling reaction of 52 with
an excess of stannylpentyne directly furnished 55 (ca. 10% yield), accompanied by
an unexpected product 56 (ca. 18% yield). Structures of both compounds were
verified by X-ray crystallography [
73
]. Apparently, 56 should be generated from 55
by the Bergman-type cyclization and subsequent cleavage of the rim bond.
Compared to metal-catalyzed functionalization of 52, decaaryl-substituted
corannulenes 57 were prepared by repetitive palladium-catalyzed arylation of
corannulene with arylboroxin through C-H activation (Scheme
15
)[
74
]. It was