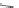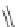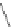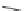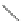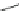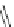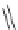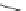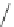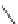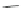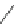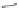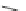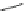Chemistry Reference
In-Depth Information
R
R
Pd(PPh
3
)
4
ArB(OH)
2
Na
2
CO
3
R, CuI
R
R
PdCl
2
(PPh
3
)
2
18
R
R
NEt
3
,90°C
or NiCl
2
dppe
AlMe
3
R
R
R=ArorSiMe
3
42
(R = Ar)
43
(R = Me)
41
R, CuI
PdCl
2
(PPh
3
)
2
Br
Br
NEt
3
,90°C
R
R
R=ArorSiMe
3
21
44
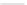
Scheme 10 Synthesis of 1,2,5,6-tetrasubstituted corannulenes [
37
-
39
]
t
-Bu
F
3
C
t
-Bu
CF
3
I
360 ºC
CF
3
t-
BuCl
AlCl
3
t-
Bu
F
3
C
68%
15%
t
-Bu
CF
3
1
t-
Bu
F
3
C
45
-CF
3
45
-
t-
Bu
Scheme 11 Synthesis of pentaalkylcorannulenes directly from corannulene [
80
,
81
]
groups generated 1,3,5,7,9-penta-
tert
-butylcorannulene (45-
t
-Bu) in a symmetrical
fashion (Scheme
11
)[
80
]. Trifluoromethylations of corannulene (1) under harsh
reaction conditions gave 1,3,5,7,9-pentakis(trifluoromethyl)corannulene (45-CF
3
),
accompanied by hexa- and hepta-substituted products [
81
]. The pure form of
45-CF
3
was obtained by HPLC separation.
1,3,5,7,9-Pentachlorocorannulene (46) provides a general route for preparing
pentasubstituted corannulenes selectively. A curious reaction between ICl and
1 gave 46 in roughly 50% yield [
34
,
49
]. At that time, the cross-coupling reactions
of aryl chlorides with various reagents were much more difficult than the
corresponding bromides. Thus, synthesis of 1,3,5,7,9-pentasubstituted corannulenes
from 46 presented several major challenges, among them overcoming the sparing
solubility, activating the chloride for substitution, and completing the reaction five
times on each molecule. Developing effective synthetic methods for coupling
reactions with 46 was an accomplishment on a par with the most difficult substrates.
Despite the expected low yields and poor reactivity, several solutions to this problem
have been found [
70
,
82
-
94
].
Very recently, 1,3,5,7,9-pentakis(Bpin)corannulene (47) was successfully pre-
pared in Scott's laboratory (Scheme
12
)[
82
]. In the presence of catalytic amounts
of [Ir(OMe)COD]
2
and 4,4
0
-dimethylbipyridyl, the reaction of corannulene with bis