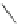Chemistry Reference
In-Depth Information
Energy
R
F
R
1
1
R: Rim; F: Flank
H: Hub; S: Spoke
H
S
D
G
‡
inv
(kcal/mol):
11.5 (R
1
=H,exptl.esti.)
9.2 (R
1
= H, B3LYP/cc-pVDZ)
10.2 (R
1
=CMe
2
OH)
11.3 (R
1
=
i
-Pr)
11.0 (R
1
=CH
2
Br)
11.2 (R
1
=Bn)
1
1'
D
G
inv
Reaction Coordinate
Fig. 1 Energy diagram and
ʔ
G
{
inv
of the bowl inversion process of corannulenes [
51
,
61
,
62
]
reduced by tetracationic tetrapyriduna-tetrabenzyna-cyclododecaphane (ExBox
4+
)
[
67
]. Experimental studies were conducted with ethylcorannulene, and its inversion
barrier was determined to be 10.8 and 8.71 kcal/mol in the absence and presence of
ExBox
4+
, respectively. The decrease in the inversion barrier (2.09 kcal/mol) deter-
mined experimentally is very close to the theoretical analysis (ca. 2.5 kcal/mol),
which suggested the results are contributed by the stabilization of the planar
transition-state structure (2.0 kcal/mol) and destabilization of the ground-state
(0.5 kcal/mol).
As the hydrogens of the
peri
positions in 1 are replaced by larger moieties, the
repulsion energy increases and
G
{
inv
decreases relative to 1 (Table
1
)[
57
,
62
].
The order of barrier heights of some 2,3-disubstituted corannulenes determined
experimentally follows oxygen (35, 9.9 kcal/mol)
ʔ
>
bromomethyl (34, 9.1 kcal/mol), and all these examples exhibit lower barriers than
other non-
peri
disubstituted corannulene derivatives, such as 36 (9.9 kcal/mol)
and 37 (10.4 kcal/mol). The lower barriers found for the
peri
-substituted com-
pounds compared to that found for the same substituents in isolated positions
shows the special contribution from
peri
X/X repulsion. Substitution at the
peri
positions as well as the 1,6-positions leads to a further reduction in the barrier,
for example compound 38 (8.7 kcal/mol). From an assumption of additivity in
steric bulk, one can assess the steric size of a
peri
substituent as being roughly
OR
>
phenyl (32, 9.4 kcal/mol)
<
¼
<
Ph
Cl
Me.
Br
Br
MeO
OMe
Br
Br
Cl
Cl
36
37
38