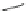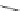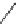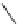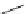Chemistry Reference
In-Depth Information
1
R
NiCl
2
(dppp)
AlMe
3
90%
O
1) NaNH
2
t
-BuOK
FeBr
3
Br
2
95%
or
1)
n
-BuLi
2) acetone
or DMF
2)
O
31
25
Br
R=Me,CHO,
CMe
2
OH
Pd
Cl
2
(PPh
3
)
2
NiCl
2
(dppp)
ArZnCl
R
R
Ar
28
-Br
30
29
X
F
AuCl
3
NIS or NCS
XeF
2
1
CH
2
Cl
2
34%
1,2-C
2
H
4
Cl
2
28
-I (99%)
28
-Cl (90%)
28
-F
Scheme 8 Synthesis of mono-substituted corannulenes from bromocorannulene [
40
,
49
-
54
]
NiCl
2
(dppp)
NaOR
ROH
PhMgBr
or AlMe
3
25%
R=(CH
2
CH
2
O)
2
CH
3
Cl
Cl
R
R
OR
OR
19
32
(R = Ph, 40%)
33
(R = Me, 90%)
35
NBS,
hv
90%
34
(R = CH
2
Br)
Scheme 9 Synthesis of 2,3-disubstituted corannulenes [
57
]
also generated corannulyne in situ. Corannulyne so generated can undergo
Pd-catalyzed [2+2+2] cyclotrimerization [
55
], as well as oligomerization [
56
].
Preparation of 2,3-disubstituted corannulene derivatives requires an alternative
synthesis (Scheme
9
)[
57
]. 2,3-Diphenylcorannulene (32) was generated by the
Ni-catalyzed Kumada coupling of 2,3-dichlorocorannulene (19) with phenyl-
magnesium bromide. Similarly, the Ni-catalyzed methylation of 19 with trimethyl-
aluminum yielded 2,3-dimethylcorannulene (33), from which the bis-
(bromomethyl) derivative 34 was obtained by benzylic bromination with
N
-bromosuccinimide. Moreover, heating 19 in diethylene glycol monomethyl