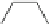Chemistry Reference
In-Depth Information
R
2
R
2
R
2
R
2
Br
Br
R
1
R
1
Method C
NaOH, dioxane, H
2
O
R
1
R
1
Br
Br
Br
2
HC
CHBr
2
66
−
96%
a
(R
1
=Me,R
2
=H)
b
(R
1
=H,R
2
= 2-Br-Ph)
c
(R
1
=H,R
2
=CF
3
)
221
20
Scheme 5 Synthesis of dibromo-substituted corannulenes derivatives by Method C [
25
,
39
,
40
]
R
1
R
1
R
1
R
1
R
1
R
1
Method D
BrH
2
C
CH
2
Br
Ni, DMF
Br
Br
60%
a
(R
1
= CO
2
Me, R
2
= H)
b
(R
1
= CO
2
Me, R
2
= Cl)
c
(R
1
= CF
3
, R
2
= H)
d
(R
1
= R
2
= CF
3
)
11
−
Br
2
HC
CHBr
2
R
2
R
2
R
2
R
2
R
2
R
2
22
23
24
Scheme 6 Preparation of corannulene derivatives 23 by Method D [
26
,
27
,
40
,
41
]
Although methods A-C are efficient in generating corannulene derivatives, they
would be problematic for sensitive functionality like the ester moiety. For example,
cyclization of precursors 22 (R
1
CO
2
Me) is doomed to failure under the reduction
conditions of Methods A and B or in the presence of aqueous base as in Method C.
A protocol developed by Sygula et al. formed the corannulene core by a nickel-
mediated intramolecular coupling of benzyl and benzylidene bromides (Method D,
Scheme
6
)[
26
,
27
]. In contrast to other methods presented above, this protocol
provides higher tolerance of functional groups, especially esters. In the absence of
ester moiety, corannulene derivatives such as 23c and 23d were obtained in low
yield [
40
,
41
]. Closure of two six-membered rings leads to completely
debrominated products 23. According to the postulated mechanism [
42
], dibromide
24 should be formed; however, it was not observed, probably due to a spontaneous
double elimination of HBr giving 23 [
26
].
The aforementioned synthetic methods allow the formation of various
corannulene derivatives in gram quantities. This scale makes the corannulene study
appropriate for dedicated programs but does not make it a suitable article of
commerce. Fortunately, corannulene (1) can now be obtained in kilogram quantities
[
43
]. This large-scale and efficient production involves a number of important
innovations, including (1) use of safer reagents, and (2) no column purification is
required; for example, the preparation of the precursor, 3,8-dimethylacenaphthe-
nequinone (6,R
2
¼
Me, Scheme
2
), from 2,7-dimethylnaphthalene is accompanied
by a substantial amount of the undesired 4,7-dimethylacenaphthenequinone, which
can easily be removed by treatment of the product mixture with the Girard's reagent.
¼