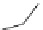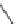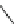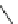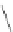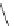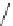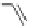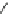Chemistry Reference
In-Depth Information
HO
OH
O
HO
2
C
1) NaBH
4
225 ºC
1
2) Pd/C,
270 ºC
4
5
Scheme 1 Synthesis of corannulene (1) based on Lawton and Barth's procedure [
17
,
18
]
O
(R
3
) H
H (R
4
)
O
R
1
R
1
R
1
R
1
O
O
R
1
R
1
7
or
R
2
R
2
base
R
3
R
4
R
2
R
2
R
2
R
2
R
1
= Me, Et, Ac, Ar
R
2
= H, Me, Et
R
3
, R
4
= CO
2
Me, Ar
6
8
9
Cl
Cl
FVP, 1100 °C
PCl
5
85%
35
−
40%
R
1
= Ac
R
2
R
4
= H
−
10
1
Scheme 2 The FVP synthesis of corannulene (1)[
20
,
21
]
acid dehydration followed by thermal decarboxylation. Sodium borohydride
reduced the ketone, and the sp
3
-hybridized framework was aromatized over palla-
dium on charcoal. This synthetic approach takes 17 linear steps to prepare
corannulene (1) in less than 1% overall yield. This classic work started the field
of Eulerean carbon surfaces; however, the lengthy synthesis and the overall low
yield limited further study of corannulene.
A few years later, high-temperature pyrolysis routes provided another way to
achieve novel strained PAH target molecules (Scheme
2
). One key reaction for the
history and development of corannulene derivatives was the synthesis of acena-
phthalene from 1-ethynylnaphthalene by Roger Brown [
22
-
24
]. This reaction
suggested the possibility of using the rearrangement of the ethynyl unit or surrogates
to a vinylidene reactive species. Larry Scott saw how to use this rearrangement to
prepare 1 in an impressively short synthesis from commercially available starting
materials, and his success sparked many others to join in the field. This practical
and efficient method developed by Scott et al. required only three steps and produced
1 in a total yield of 26% [
20
,
21
]. In the first step, the three starting materials,
i.e., 6 (R
2
H), 2,4,6-heptanetrione (7,R
1
Ac), and norbornadiene, were heated with
the catalyst, glycine, in toluene (Scheme
2
). The Knoevenagel condensation
between 6 and 7 afforded the cyclopentadienone derivative 8 (R
1
¼
¼
Ac, R
2
H), which
underwent Diels-Alder reaction with norbornadiene to give 7,10-diacetylfluoranthene
9 (R
1
¼
¼
Ac, R
2
¼
¼
H) with loss of carbon monoxide and cyclopentadiene.