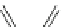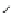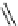Chemistry Reference
In-Depth Information
16
4
13
1
16d
16a
16c
1
6b
90
Fig. 27
Structure of 1,4,13,16-tetramethylbenzopentahelicene
90
[
83
]
1. 2
-BuLi
2. FeCl
2
.
2 THF
t
Fe
(
M
)-(
_
)-
92
,60%
(
M
)-(
_
)-
91
Scheme 19
Synthesis of optically active helical ferrocene (
M
)-(
)-
92
[
84
]
Computation using the semiempirical AM1 method shows a C16a-C16b-C16c-C16d
torsional angle of 34.3
and a heat of formation for racemization of 33.7 kcal/mol,
substantially higher than those of the derivatives without substitutions at C1 and C16
positions.
Optically active dibenzo[
c
,
g
]phenanthrene (
M
)-(
)-
91
bearing two fused
cyclopentadienyl rings was synthesized by the photodehydrocyclization method
with chirality being controlled during the cyclization process [
84
]. The optically
active helical ferrocene (
M
)-(
)-
92
was obtained by treatment of (
M
)-(
)-
91
with
tert
-butyllithium followed by FeCl
2
·2THF (Scheme
19
).
4.2
1,14-Diaryldibenzo[
c
,
g
]phenanthrenes
The photodehydrocyclization reaction was employed to produce 14,15-
diphenyldibenzo[
f
,
j
]picene (
93
) (Fig.
28
) in 0.8% yield [
85
]. The mass and
1
H
NMR spectra were used to support the structure assignment.
The diindeno-fused 1,14-diphenyldibenzo[
c
,
g
]phenanthrene
97
was synthesized by
the cascade cyclization reactions of the benzannulated enediyne
96
(Scheme
20
)[
76
].
As in the case of
85
, the X-ray structure of
97
(Fig.
29
) shows profound twist with a
57.8
acute dihedral angle between the mean planes of rings A and E. Again, each
phenyl substituent is oriented at a 60.2
angle from the benzene ring to which it is
attached but is in roughly parallel orientation to the opposite side of the twisted dibenzo
[
c
,
g
]phenanthrene framework with a distance of ca. 3.00
˚
at the closest point.