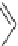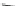Chemistry Reference
In-Depth Information
13
14
3
2
12
1
A
E
11
4
14d
14a
14b
14c
B
D
C
86
Fig. 25
Structure of dibenzo[
c
,
g
]phenanthrene [
77
]
R
R
87a
:R= Br
87b
: R = CHO, 69%
87c
:R= CH
2
OH, 85%
Fig. 26
Structures of 1,14-dimethyldibenzo[
c
,
g
]phenanthrenes [
81
,
82
]
O
O
O
NaH
87c
p
-Ts(OCH
2
CH
2
)
5
O
p
-Ts
O
O
88
O
89
,26%
Scheme 18
Synthesis of benzo[
c
]phenanthrene
89
bearing a crown ether [
81
]
at ambient temperature [
77
]. A computational study indicates that substitutions at C1
and C14 positions cause substantially higher activation barriers [
79
]. The X-ray
structures of several substituted dibenzo[
c
,
g
]phenanthrenes have been reported [
80
].
While 1,14-dimethyldibenzo[
c
,
g
]phenanthrene has not been synthesized,
1,14-dimethyl derivative
87a
(Fig.
26
) has been prepared by the photodehydro-
cyclization reaction of a stilbene-like precursor [
81
,
82
]. Treatment of
87a
with
n
-butyllithium followed by DMF produced dialdehyde
87b
in 69% yield. Reduction
of
87b
with lithium aluminum hydride gave diol
87c
in 85% yield. On exposure to
sodium hydride and bistosylate
88
, diol
87c
was converted to
89
bearing a crown
ether moiety (Scheme
18
). Resolution of
89
to optically pure (
M
)-(
)-
89
and (
P
)-
(+)-
89
was achieved with a chiral HPLC column. These 1,14-disubstituted dibenzo
[
c
,
g
]phenanthrenes were optically stable, showing no optical change after refluxing
in ethanol for 24 h. Selective chiral recognitions of one of the enantiomers of
methyl phenylglycinate hydrochloride, 1-phenylethylamine hydrochloride, and 1,2-
diphenylethylamine hydrochloride were observed.
Tetramethyl-substituted benzopentahelicene
90
(Fig.
27
) was synthesized by the
Diels-Alder reaction between a diene and benzyne followed by dehydrogenation [
83
].