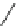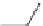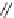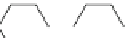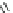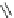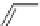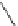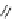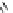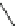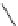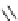Chemistry Reference
In-Depth Information
R
1
R
1
R
2
13
14
R
1
R
1
R
2
ZnCl
2
microwave
5 min
N
N
56
+
HO
O
O
OH
59a
:R
1
=R
2
= H, 83%
59b
:R
1
=Me,R
2
= H, 84%
59c
:R
1
=H,R
2
= Me, 34%
58
Scheme 12
One-pot synthesis of dibenzo[
b
,
j
][4,7]phenanthrolines containing a trimethylene
bridge [
57
]
N
O
O
2
PPh
3
n
-C
8
H
17
N
N
R
R
N
N
61
refluxing
p
-xylene
N
N
60
62
R
R
HN
NH
N
N
63
,R=
n
-C
8
H
17
, 66%
Scheme 13
Synthesis of diindolophenanthroline
63
[
59
]
mixture of (
S
)-(
M
)/(
R
)-(
P
)-
59c
was produced exclusively. Since rapid equilibration
between the two diastereomeric pairs can be expected, the exclusive formation of
(
S
)-(
M
)/(
R
)-(
P
)-
59c
can only be attributed to their higher thermodynamic stability.
The cascade cyclization reactions of the benzannulated enyne-carbodiimide
62
,
prepared in situ by the aza-Wittig reactions between 1,4-phenylene diisocyanate
(
60
) and 2 equivalents of iminophosphorane
61
, produced diindolophenanthroline
63
(Scheme
13
)[
59
]. The reaction mechanism is similar to that described for the
benzannulated enyne-allene
36
in Scheme
6
.The
1
H NMR spectrum of
63
shows
two sets of signals with equal intensity at
d
4.00 and 3.74, indicating the
diastereotopic relationship of the two benzylic hydrogens on the same carbon
atom and manifesting the presence of a helical twist. These two signals remain
well separated and exhibit no line broadening at 110
C, indicating a relatively slow
rate of racemization on the NMR time scale.