Chemistry Reference
In-Depth Information
Br
Br
Br
Br
33
Br
n
BuLi
Br
Br
n
BuLi
Br
59
61
1:1
Br
Br
Br
Br
62
n
BuLi
n
BuLi
2:1
60
63
Chloranil
1. OsO
4
,
NMO
2. TEMPO
NaOCl
n
64
:n=0
65
:n=1
O
O
n
O
O
66
:n=0
67
:n=1
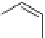
Fig. 28 Synthesis of photoprecursors for octacene (66) and nonacene (67)[
60
]
substance. Motivated by the success of the photobisdecarbonylation strategy of
bridged
-diketones in the synthesis of heptacene and smaller acenes under matrix
isolation conditions [
22
,
48
], we have developed a route to octacene and nonacene
under matrix isolation conditions (Fig.
28
)[
60
]. The key was to install two
α
α
-diketone bridges as in 66 and 67 to avoid having acene units larger than
anthracene as otherwise problems associated with stability and solubility may
hinder the synthesis of the required photoprecursors. The final step (Fig.
28
), the
oxidation of the diols to the
-diketones, proved to be most difficult and was
achieved by Anelli oxidation [
60
].
Matrix isolation of the nonacene photoprecursor 67, followed by irradiation
α
(
395 nm), produced an UV/vis spectrum that showed the typical vibrational
structure of the
p
band in the region of a hexacene derivative (Fig.
29a
). This
behavior is in agreement with the photochemical decarbonylation of one of the two
α
λ
>
-diketone bridges.
Further
305-320 nm) resulted in
decrease of the band associated with the hexacene derivatives into a set of new
irradiation at shorter wavelengths (
λ ¼