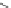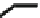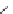Chemistry Reference
In-Depth Information
1. NaBH
4
, EtOH, rt
2. HBr, AcOH, reflux
3. PPh
3
, toluene reflux
O
O
O
PPh
3
Br
64%
98
99
NBS
benzoyl peroxide
CCl
4
, reflux
40%
1. LiOEt/EtOH, DMF, -10˚C
2.
hv
, benzene, 5˚C
6%
100
Br
Br
O
O
TiCl
3
(DME)
1.5
Zn·Cu,
DME, reflux
8%
IBX
DMSO, 65˚C
Br
Br
O
O
Br
Br
37%
O
O
[6.8]
3
cyclacene
101
102
Fig. 47 Glieter's synthesis of [6.8]
3
cyclacene [
74
]
Though the carbon skeleton was assembled in these two attempts, so far there is
no successful synthesis of any [6]cyclacene. This enticing molecule—the shortest
possible carbon nanotube, with a highly strained skeleton and double trannulene
system—will surely not remain an unsolved synthetic problem indefinitely.
6.3
[6.8]Cyclacene
The instability of the [6]cyclacenes is due both to their highly distorted geometry
and their calculated singlet-triplet gaps, which narrow with increasing size, falling
to near-degeneracy at [6]
8
cyclacene. To circumvent these unfavorable properties,
Glieter employed a moving target approach, proposing that a belt with alternating
fused benzene and cyclooctatetraene rings would posses more stable electronics.
The boat conformation of the cyclooctatetraene moieties should both alleviate
strain and contort the
-system to increase the filled-unfilled orbital gap [
74
].
This stability was confirmed by the synthesis of [6.8]
3
cyclacene via a Wittig
macrocyclization and McMurry coupling sequence (Fig.
47
).
Dialdehyde 96 was converted to the corresponding phosphine salt 99 and dilute
macrocyclization followed by irradiation of the resulting
E/Z
isomer mixture
coalesced into the all-
Z
101. Following bromination and oxidation, McMurry
conditions closed down the remaining eight-membered rings to offer
[6.8]
3
cyclacene with the majority of hexaaldehyde 102 presumably oligomerizing
in the reaction mixture [
74
].
This cyclacene was found to have
D
3
h
symmetry with boat-like cycloocta-
tetraenes and almost planar benzene rings (Fig.
48
)[
74
].
π