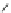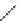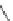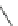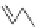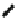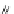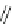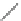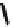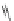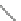Chemistry Reference
In-Depth Information
O
O
O
O
H
H
91
H
H
O
O
O
17%
H
H
H
H
92
O
O
93
1. TiCl
4
, LiAlH
4
2. Ac
2
O, HCl
56%
94
Fig. 45 Stoddart's attempted synthesis of [6]
12
cyclacene [
71
]
R
R
reflux
dioxane
R
R
R
R
95
O
O
R
R
69%
O
O
O
O
97
R =
n
-hexyl
O
O
96
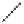
Fig. 46 Cory's attempted synthesis of [6]
8
cyclacene [
72
]
6.2 Towards the Synthesis of [6]
8
Cyclacene
In 1996, Cory reported attempts to access [6]
8
cyclacene from macrocycle 97
(Fig.
46
). Building upon the macrocyclization work of Schl
¨
ter, this molecule
was easily synthesized from the reaction of flexible bisdiene 95 with rigid and
planar anthrodiquinone 96 [
73
].
In this way Cory avoided issues of diastereoselectivity and was able to isolate 97
in 69% yield. Alkyl chains were used to avoid the problem of solubility encountered
by Stoddart [
72
]. Several attempts were made to convert unstrained 97 into
[6]
8
cyclacene including a host of oxidation and deoxygenation conditions, but
formation of the cyclacene was never observed [
72
]. Again, only pathways with
oxidative and acidic conditions in the final steps were explored. As previously
mentioned, the desired product is likely very unstable in such conditions.