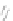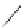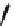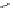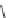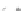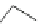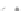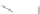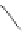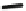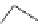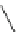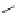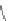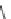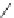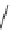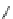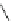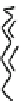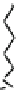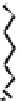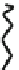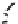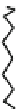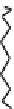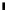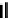Chemistry Reference
In-Depth Information
Me
H
Me
Me
Me
1. MeMgBr, CuBr·SMe
2
DMI, THF
2. NH
4
Cl/H
2
O
Me
92%
C
60
86
1.
t
-BuOK, THF
2. TsCN, PhCN
63%
Me
CN
Me
CN
Me
Me
Me
Me
Me
Me
Me
Me
1. PhMgBr, CuBr·SMe
2
DMI, THF
2. NH
4
Cl/H
2
O
14%
Ph
Ph
Ph
Ph
Ph
H
87
88
1. Li
+
[C
10
H
10
]
-
, PhCN
2. NH
4
Cl/H
2
O
82%
OH
Me
Me
H
Me
O
Me
O
Me
Me
Me
Me
Me
Me
KH, THF
under air
42%
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
H
Ph
O
OH
89
90
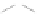
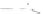
Fig. 43 Synthesis of substituted [10]cyclophenacene by modification of C
60
fullerene [
67
,
68
]
Fig. 44 Cyclacene
envisioned as the unit-cycle
of a zigzag nanotube (double
bonds omitted for clarity)
of longer acene moieties by way of spontaneous decomposition in the reaction
media. Insolubility and large energy requirements due to strain buildup may have
also prevented the oxidation of eight additional carbons and conversion to
[6]
12
cyclacene [
71
].