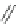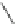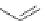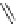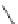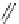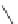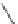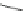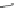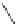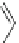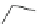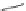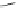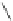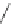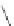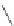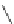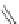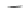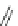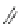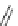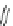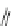Chemistry Reference
In-Depth Information
MOMO
OMOM
Br
O
B(OH)
2
4 step
s
MOMO
OMOM
58a
B(OH)
2
Br
O
Excess
Bpin
Bpin
Br
Br
85
[14,4]CPPy
Macrocycle
48%
N
N
79%
PdCl
2
(dppf)
Na
2
CO
3
n
-Bu
4
NBr
Toluene/H
2
O
Reflux
Br
Br
PdCl
2
(dppf)
Na
2
CO
3
n
-Bu
4
NBr
Toluene/H
2
O
Reflux
N
N
N
N
NaHSO
4
·H
2
O
o
-chloranil
m
-xylene/DMSO
150˚C
MOMO
OMOM
MOMO
OMOM
84
N
N
N
N
[14,4]CPPy
56%
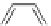
Fig. 42 Synthesis of [14,4]CPPy [
46
]
6.1 Towards the Synthesis of [6]
12
Cyclacene
Two notable attempts at synthesizing cyclacenes have been made, the first by
Stoddart towards [6]
12
cyclacene in 1992 and the second by Cory towards
[6]
8
cyclacene in 1996 [
71
,
72
]. Stoddart was simply able to build the complex
macrocycle Kohnkene 93 in 17% total yield by the sequential reaction of 2 equiv.
each of carefully selected but easily synthesized bisdiene 91 and dienophile 92
(Fig.
45
)[
71
].
The electron densities favor only the Diels-Alder addition to the bottom face of
the
exo
-diene and the top face of the bicyclic dienophile moieties, thereby yielding
93 under high temperature and pressure. Deoxygenation with low-valent titanium
followed by dehydration with acetic anhydride yielded the partially-saturated
cyclophane 94. Given the propensity of linear acenes to oxidative decomposition,
the late stage, harsh acidic oxidation, in this synthesis likely prohibited the formation