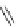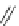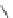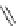Chemistry Reference
In-Depth Information
PtCl
2
(cod)
(1.0 eq.)
CsF (6.0 eq.)
Hex
Hex
X
Pt(cod)
X
THF
reflux 24 h
4
Hex
Hex
78
74%
B
2
pin
2
(2.2 eq.)
PdCl
2
(dppf)
·
CH
2
Cl
2
(3 mol%)
KOAc (3.0 eq.)
Toluene 80-100˚C 33 h
91%
76
(X = Br)
Toluene
rt 30 min
100˚C 24 h
PPh
3
(40 eq.)
77
(X = Bpin)
Hex
Hex =
n
-hexyl
Bpin = boronic acid pinacol ester
4
Hex
[4]CC
79
94%
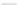
Fig. 39 Synthesis of a chiral nanohoop with atropisomerism [
39
]
We have so far focused on single-stranded molecular belts which all display
some degree of structural rotation. It is easy to envision molecular belts with two or
more strands and a rotationally constrained ladder-type backbone. Discussion of
these two-stranded molecular belts follows.
5
“Top-Down” Synthesis of [10]Cyclophenacene
One approach to the synthesis of double-stranded aromatic belts is the degradation
of higher-order carbon materials with atomistic accuracy. There is only one exam-
ple of this type in the literature. Nakamura and coworkers developed controllable
fullerene chemistry and were able to synthesize successfully a [10]cyclophenacene
derivative by the careful selective substitution of the north and south poles of C
60
fullerene (Fig.
43
)[
65
-
68
].
The north pole of the fullerene was methylated by reaction with methyl cuprate.
This methylated fullerene, 86, was then converted to cyano-fullerene 87 to prevent
formation of the cyclopentadienyl anion by deprotonation in subsequent steps.
Derivative 87 was treated with a phenyl cuprate to yield phenyl-substituted 88
with an electronically isolated [10]cyclophenacene. Removal of the cyano group
and oxidation gave the penta-oxygenated derivative 90, which could be easily