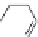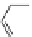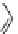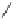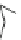Chemistry Reference
In-Depth Information
Br
Br
1.
n
BuLi
2. CeCl
3
, LiCl
3. cyclohexane-1,4-dione
4. MOMCl,
i
-Pr
2
NEt
RO
OR
Br
Ni(cod)
2
(2 eq.)
bpy (2 eq.)
[9]CPP
65%
THF reflux 24 h
RO
RO
OR
NaHSO
4
(20 eq.)
m
-xylene
DMSO
reflux in air
Br
RO
OR
RO
60
56
81%
OR
OR
R = MOM
RO
OR
[12]CPP
24%
RO
OR
RO
OR
61
Fig. 27 Nickel “shotgun” synthesis of [9]CPP and [12]CPP [
41
,
42
]
in a 7+7, 7+8, or 8+8 fashion to offer 2.0, 2.2, and 2.5 mg of [14]CPP, [15]CPP, and
[16]CPP respectively after acidic aromatization. The authors propose that chair-
flipping accounts for increased flexibility, facilitating the 7+8 macrocyclization [
36
].
Itami went on to report a new synthesis of [12]CPP based on the “shotgun”
homocoupling of dihalide 56 with bis(cyclooctadiene)nickel(0) in the presence of
2,2
0
-bipyridyl. This new approach circumvented the synthesis of a boronate and
also the need for palladium catalysis, albeit stoichiometric nickel is required
(Fig.
27
). With these synthetic advantages, Itami was able to synthesize 61, the
macrocyclic precursor to [12]CPP on a gram scale. In addition, the authors report
that with this synthesis they were able to produce 0.5 g in total of [12]CPP from
combined reaction products [
36
].
At the time of publication, the “shotgun” coupling in this synthesis was reported
only for the [12]CPP macrocycle. However, upon investigation of what was
originally thought to be linear oligomeric byproducts, it was found that the triangu-
lar nine-membered macrocycle 60 also forms in similar yields to the [12]CPP
precursor (Fig.
27
)[
42
].