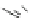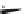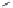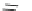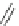Chemistry Reference
In-Depth Information
SnMe
3
Pt
Pt
Pt(cod)Cl
2
THF, reflux
57%
SnMe
3
Pt
Pt
53
54
dppf
CH
2
Cl
2
, rt
91%
dppf
dppf
Pt
Pt
Br
2
Toluene, 95˚C
49%
[8]CPP
Pt
Pt
dppf
dppf
55
Fig. 25 Yamago's synthesis of [8]CPP [
63
]
that such a strained hydrocarbon, which was envisioned decades ago, has become
commercially available so shortly after its first synthesis. It seems evident, therefore,
that interest in these structures is not limited to synthetic chemists.
In 2010, Yamago et al. entered the field of carbon nanohoop synthesis, reporting
the challenging synthesis of [8]CPP, the smallest and most strained CPP at the time
of publication. Unlike Itami and Jasti, who relied on masked aromatic rings to
relieve strain in the macrocyclic CPP precursors, Yamago employed an organome-
tallic approach (Fig.
25
). By reacting 4,4
0
-bis(trimethyl)stannylbiphenyl 53 with
dichloro(cycloocta-1,5-diene)platinum(II), Yamago was able to generate square-
shaped macrocycle 54 with very little strain energy [
63
].
After ligand exchange to 1,1
0
-bis(diphenylphosphino)ferrocene, (dppf) reductive
elimination with bromine led cleanly to 2.0 mg of [8]CPP in three straightforward
steps. This reductive elimination builds in an incredible 74 kcal/mol of strain energy
in a single reaction. Yamago observed that this new, smaller cycloparaphenylene
followed the optical trend observed by Jasti having an absorption maximum at
338 nm and an even larger absorbance-emission shift of 200 nm [
63
].