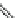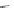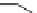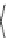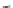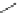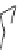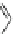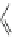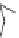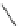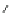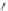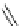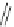Chemistry Reference
In-Depth Information
I
O
1.
n
BuLi
THF, -78˚C to rt
2. MOMCl,
i
Pr
2
NEt
CH
2
Cl
2
, rt
I
+
excess
I
MOMO
47%
I
O
PdCl
2
(dppf)
NaOH
1,4-dioxane/H
2
O
60˚C
OMOM
1.
n
BuLi
THF, -78˚C to rt
2. B
2
pin
2
, PdCl
2
(dppf)
KOAc, DMSO, 80˚C
49
Bpin
39%
Bpin
HO
OH
OH
OMOM
50
HO
OMOM
I
OMOM
50
, Pd(OAc)
2
X-Phos, NaOH
1,4-dioxane/H
2
O 80˚C
OMOM
I
MOMO
OH
MOMO
OH
MOMO
OH
52
51%
MOMO
OH
p
TsOH
m
-xylene, 150˚C
51
81%
[12]CPP
62%
Fig. 24 Itami's synthesis of [12]CPP [
35
]
molar excess of 49 with 50 under appropriate palladium coupling conditions
favored the formation of 9-membered diiodide 51. Dilute coupling of 51 with
bisboronate 50 yielded selectively the 12-membered macrocycle (Fig.
24
).
To aromatize the cyclohexane rings in this CPP precursor, Itami employed harsh
but effective microwave conditions using stoichiometric
para
-toluenesulfonic acid
at 150
C to achieve the sequential deprotection of the alcohols, acidic elimination,
and oxidation of the resulting cyclohexadienes to aryl rings. Using this motif, Itami
obtained 4.1 mg of [12]CPP alone [
35
]. This methodology has recently been
employed by Tokyo Chemical Industry, who now offers [12]CPP of 90% purity
for $999.00 per 10 mg (TCI, product number C2449). It is an incredible achievement