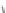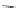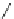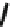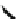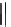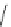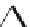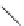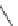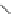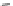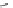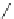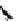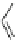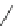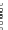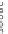Chemistry Reference
In-Depth Information
I
Bpin
H
3
CO
H
3
CO
I
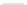
a) i. nBuLi, THF,
-78°C
ii. benzoquinon
e
i. nBuLi, THF,
-78°C
44
(34%)
45
(82%)
b) i. NaH,THF, 0°C
ii. MeI
ii.
Bpin
O
H
3
CO
I
H
3
CO
-78°C
I
Bpin
OCH
3
H
3
CO
44
Pd(PPh
3
)
4
Cs
2
CO
3
46
, n = 2 (2%)
47
, n = 3 (10%)
48
, n = 5 (10%)
+
Toluene/MeOH (10:1)
80°C
45
H
3
CO
OCH
3
n
lithium
naphthalenide
Macrocycles
46
,
47
, and
48
THF, -78 °C
n
n = 5, 8, 14
[9], [12], [18]CPP
43%, 52%, 36%
Fig. 21 The first synthesis of [9]-, [12]-, and [18]cycloparaphenylene [
32
]
Macrocycle
n
[9]-, [12]-, [18]CPP
OMe
Lewis acidic
reagents
Lithium Naphthalide
43%, 52%, 36%
undesired products
e
-
-H
+
Li
e
-
H
OMe
MeO
MeO
OMe
Fig. 22 Aromatization reaction and proposed mechanism [
32
]
at low temperatures (total calculated strain energy for [9]CPP: 69 kcal/mol). Using
this procedure, Jasti synthesized 1.8, 4.2, and 1.6 mg of [9]CPP, [12]CPP, and [18]
CPP for the first time.