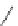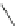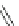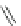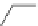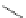Chemistry Reference
In-Depth Information
1. Br
2
, CHCl
3
2.
t
-BuOK, Et
2
O
m
12
: m = 1 (85%)
13
: m = 3 (85%)
n
9
: n = 1 (30-40%)
10
: n = 3 (15%)
11
: n = 5 (5%)
Fig. 7 Kawase's synthesis of [6]CPPA and [8]CPPA [
16
]
Br
Br
t-
BuO
Br
Br
Br
2
t-
BuOK
CHCl
3
Et
2
O
Br
Br
O
t-
Bu
Br
Br
15
30-40% overall
9
14
Fig. 8 Failed conversion of 9 to [4]CPPA [
14
]
3.2 CPPAs as Novel Host Molecules
Having overcome the challenge of [6]CPPA and [8]CPPA with his landmark synthe-
sis, Kawase improved upon these methods to prepare large quantities of [6]-[9]
CPPA. With ample material in hand, the Kawase lab sought to exploit the unique,
electron-rich cavity present in these rigid molecular belts [
16
]. Kawase showed for the
first time that CPPAs can form all-hydrocarbon inclusion complexes with appropriate
guests [
17
]. By co-crystallizing [6]CPPA and [8]CPPA with hexamethylbenzene and
toluene respectively, crystal structures of a 1:1 12 ·HMBand1:413 · tol complexes
were obtained (Fig.
9
).
Though the host-guest interaction in both of these complexes was quite weak,
with the toluene complex efflorescing at room temperature, the improved air-
stability of the complexes as compared to the CPPAs indicated a favorable electronic
interaction within the CPPA cavities [
17
]. The authors attribute this affinity to the
electron-rich nature of the molecules' interior. Furthermore, important crystallo-
graphic information about the diameters of these molecular belts was available for
the rational design of stronger host-guest complexes. With preliminary proof of the
CPPAs' host activity, and in light of the then recent discovery of carbon nanotube
fullerene peapod complexes, Kawase correctly ascertained that [6]CPPA was
approximately the right size to host fullerene C
60
. Indeed, mixtures of 12 and C
60