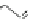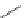Chemistry Reference
In-Depth Information
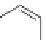
Fig. 1 PAH isomers and p-orbital orientations of C
48
H
24
: graphene-like kekulene (
top
) and
nanotube-like [6]
12
cyclacene (
bottom
)
H
H
H
hv
mass spectrometry
H
H
cyclo[18]carbon
(observed)
H
1
Fig. 2 The first synthesis of a cyclo[
n
]carbon by Diederich et al. [
4
]
closure through standard chemical cyclodehydrogenation (e.g., Sch¨ ll, etc.) which
only led to polymerization. Flash vacuum pyrolysis at 800
C, however, yielded
40% of the interesting rearrangement product 5 with a convoluted structure and a
calculated ground state energy 52 kcal/mol more stable than that of the picotube
(Fig.
5
)[
8
].
The picotube did prove to be susceptible to Friedel-Crafts functionalization to
prepare chiral derivatives [
9
]. The authors propose this as a general method for the
functionalization of carbon nanotubes. The cavity of these picotubes is quite small
for the encapsulation of hydrocarbons as is seen with cycloparaphenylenea-
cetylenes and cycloparaphenylenes, but it has been calculated to be an appropriate
guest for a (9,9) carbon nanotube (CNT), though empirical data has not yet been