Chemistry Reference
In-Depth Information
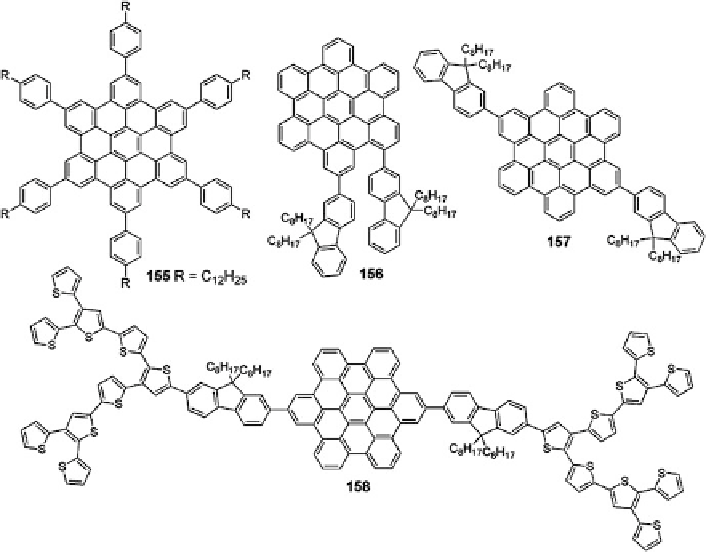
Fig. 30 HBC derivatives used in organic electronics [
113
-
116
]
material by creating an insulating domain around the
stacked columnar core.
Compounds 156 and 157 exhibited pronounced self-assembly properties and good
device results. For instance, a field-effect mobility of 2.8
p
-
p
10
3
cm
2
V
1
s
1
and
a power conversion efficiency of 1.5% were achieved for 157, which are fairly
impressive for HBC-based materials [
114
,
115
]. In subsequent studies, a series of
thiophene dendrons were introduced to extend the effective conjugation to produce
a thiophene-fluorene co-oligomer substituted HBC 158, which showed a broader
absorption between 250 and 550 nm. The corresponding BHJ devices showed good
performance with power conversion efficiency of 2.5%. This is attributed to the
enhanced light harvesting property as well as the formation of ordered morphology
in solid state induced by the self-assembly of the HBC molecules [
116
] (Fig.
30
).
4.2 All-Benzenoid PAHs Larger than HBC
Taking advantage of FeCl
3
or Cu(OTf)
2
-AlCl
3
mediated oxidative cyclodehydro-
genation reactions, larger all-benzenoid PAHs with variable shapes and sizes are
accessible. Increasing the size of the highly planar HBC core is predicted to
improve the
p
-
p
intermolecular interaction due to the large overlap of the
p
-surface