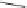Chemistry Reference
In-Depth Information
Fig. 17 The [4+4]
photodimer of pentacene (38)
38
LDI-TOF measurements [
33
]. The energy of dimerization of the substituted
compounds is likely smaller than that of the parent acenes. It is not clear whether
the dimer mass of heptacene observed is indeed due to the presence of (a structur-
ally not characterized) heptacene dimer or to laser induced breaking of heptacene
oligomers and polymers, but it is noted that the monomer mass was not observed
[
22
]. A recent computational study by the Bendikov group shows that the dimer-
ization of heptacene to the [4 + 4] dimer, a thermally forbidden reaction, is strongly
exothermic [
53
].
A strongly bound (
E
bind
¼
24.4 kcal mol
1
; M06-2X/6-31G* level of theory)
van-der-Waals dimer (7-complex in Fig.
18
) exists that presumably forms without
barrier [
53
]. The barrier for formation of the covalent dimer (7M
1
in Fig.
18
) from
the van-der-Waals dimer (via transition structure 7T
1
in Fig.
18
) is 12.3 kcal mol
1
at M06-2X/6-31G* [
53
]. This is below the energy of two separated heptacene
molecules. Hence, the energy gained by complex formation is enough to surmount
the barrier for dimerization. One should note that the description of dispersion
interactions in the anthracene dimer is problematic with density functional
methods, even if empirical dispersion corrections are included [
54
]. The M06-2X
functional has been shown to perform well for a number of systems, but how
reliable the data are for heptacene dimerization is not clear at this time. Another
problem for the reliable computation of heptacene dimerization may arise from the
triplet instability of the spin-restricted Kohn-Sham (RKS) description of the
heptacene molecule [
55
].
3.2 Substituted Heptacenes
3.2.1 Silylethynyl Substituted Heptacenes
The first unambiguous report of a substituted heptacene was provided by Payne
et al. in 2005 [
37
]. The authors synthesized silylethynyl substituted heptacenes
similar
to hexacenes: nucleophilic
addition of
lithium(silylacetylide)
to
heptacenequinone 39 followed by reduction of the diol by SnCl
2
(Fig.
19
)[
37
].
As with hexacenes, the
i
-Pr group (TIPS) was too small to stabilize the
heptacene. Even the
t
-Bu group (TTBS) resulted in a heptacene derivative that
was described as being only marginally stable. Heptacene 40b decomposed within a
day in a rigorously oxygen-free solution, and even faster when the solution was