Chemistry Reference
In-Depth Information
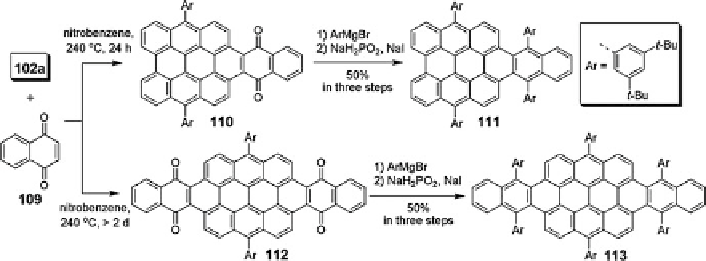
Scheme 11 Synthesis of laterally extended bisanthenes [
89
]
The synthetic approach taking advantage of Diels-Alder reaction makes it
possible to extend the
-system further by using different dienophiles. For example,
using 1,4-naphthaquinone 109 as dienophile led to bisanthene adducts 110 and 112,
whereupon nucleophilic addition of Grignard reagents followed by reductive aro-
matization gave compounds 111 and 113 (Scheme
11
)[
89
]. Early studies by M¨ llen
and coworkers revealed that
bay
-annulation of rylenes leads to hypsochromic shifts
of absorption compared to their parent rylene molecules. For this system, a slight
bathochromic shift was observed compared with
meso
-substituted bisanthenes
owing to the additionally higher conjugation along the long molecular axis. The
emission spectra of 111 and 113 showed mirror symmetry with their absorption
spectrum, and relatively moderate photoluminescence quantum yields of 0.45 and
0.38 were measured for dyes 111 and 113, indicating a potentiality of these
compounds as NIR organic light-emitting diodes (OLEDs).
Fusion of three anthracene units leads to teranthene, which is a longer homo-
logue of bisanthene and a big challenge in terms of synthetic difficulty and stability
issues. Recently, the first teranthene derivative 119 was obtained in Kubo's group
[
90
]. As shown in Scheme
12
, nucleophilic addition of lithium reagent 114 to 1,5-
dichloroanthraquinone 115 followed by reductive aromatization with NaI/
NaH
2
PO
2
gave the teranthryl derivative 116. Cyclization combined with demeth-
ylation of 116 was carried out with KOH/quinoline to give the partially ring-closed
quinone 117 which was treated with mesitylmagnesium bromide in the presence of
CeCl
3
and further underwent reductive aromatization to generate partially cyclized
compound 118. The last cyclization was promoted by DDQ/Sc(OTf)
3
followed by
quenching the reaction with hydrazine to give 119 as a dark-green solid. Compound
119 exhibited moderate stability in solution with a half-life of around 3 days upon
exposure to air at room temperature. Like other open-shell PAHs with singlet
biradical ground state, the
1
H NMR spectrum showed temperature dependence
with a line broadening at room temperature.
X-ray analysis revealed an effective
D
2h
symmetry for the teranthene core, with
two mesityl groups nearly perpendicular (Fig.
27
). A prominent biradical character
was also reflected by the geometry and bond length of the teranthene in comparison
p