Chemistry Reference
In-Depth Information
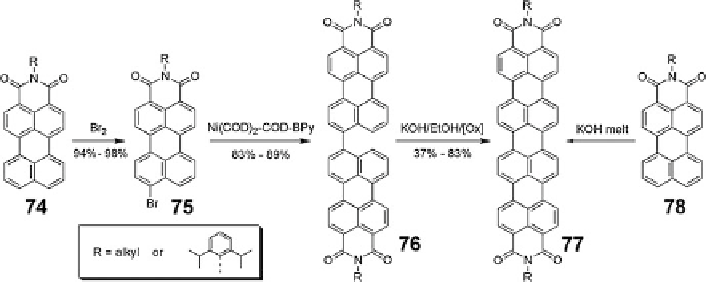
Scheme 7 Two synthetic routes to quarterrylene bisimides 77 [
69
,
70
]
then obtained by heating 76 in molten KOH in the presence of an added oxidizing
agent. Enhanced solubility can be realized by nucleophilic substitution with bulky
phenoxy groups in the bay region [
69
]. In parallel, Langhals reported a one-step
dimerization from perylene monoimide 78 inspired by the formation of perylene
bisimide from naphthalene monoimide. Reaction of 78 in alkali melt proceeded to
give quaterrylene bisimide with swallow tail substituents [
70
].
The pursuit of higher order rylene bisimides continued and the next two
members in this family, pentarylene bisimide 73d and hexarylene bisimide 73e,
were synthesized in M¨ llen's group on the basis of two different synthetic methods
(Scheme
8
)[
71
]. The first construction concept was called the “nitronaphthalene
method” in which one nitronaphthalene unit was attached to a perylenedicar-
boximide, whereupon the nitro group could be reduced and transformed into iodo
group to afford precursor 83. Subsequently, homocoupling of 83 or Suzuki coupling
with perylenedicarboximide 79 yielded 82b and 82a which, upon cyclization,
generated hexarylene bisimide 73e and pentarylene bisimide 73d. This method
generally suffered from low overall yield due to the low yield in Sandmeyer
reaction, so an alternative method named the “bisbromorylene method” was devel-
oped in which two perylenedicarboximides 79 were coupled to a bisbromo-
naphthalene 80a or bisbromoperylene 80b and fused in a one-step or two-step
sequence to form the corresponding compounds 73d and 73e. The absorption
maxima of 73d and 73e were located at 877 and 930 nm, respectively, and these
two dyes had extremely high extinction coefficients (up to 235,000 M
1
cm
1
for
73d and 293,000 M
1
cm
1
for 73e), which are the highest values reported among
all the known organic dyes in the NIR spectral region. Despite relatively low band
gaps, 73d and 73e displayed remarkable stability and their solution remained the
same over weeks in sunlight. More importantly, nearly no absorption for 73d and
73e could be detected in the visible region. All of the outstanding properties
mentioned above qualified them as promising NIR dyes for many technological
applications, such as security printing.