Chemistry Reference
In-Depth Information
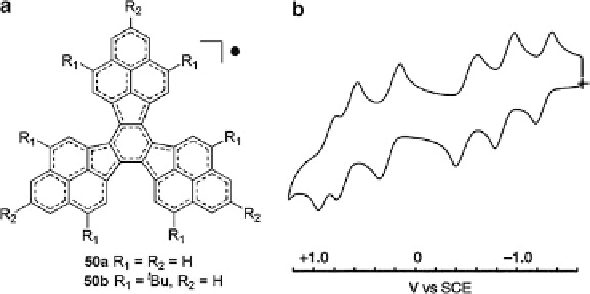
Fig. 17 (a) Structure of trisphenalenyl radical 50;(b) cyclic voltammogram of 50b [
55
,
56
]
in air. The neutral radical gave six reversible one-electron redox waves (Fig.
17b
),
providing evidence for the formation of stable monovalent, divalent, and trivalent
species, which is one of the rare examples of compounds with six-stage amphoteric
redox behavior.
2.2 Triangulene-Based Triplet Biradicals
The higher order analogue of phenalenyl is the
C
3
-symmetric polycyclic hydrocar-
bon 1 known as triangulene, which represents the most fundamental non-Kekul
´
polynuclear benzenoid compounds with at least two carbon atoms not participating
in the double bond network, giving rise to an open-shell electronic structure.
According to theoretical calculations, a triplet ground state is expected due to the
topological degeneracy of non-bonding molecular orbitals (NBMOs) with a non-
disjoint nature [
57
]. Theoretical studies also predicted large spin densities at the
edge sites, indicating its high reactivity in the neutral state. Notably, it is predicted
that the triangulene system can lead to formation of a
-stacked radical polymer
with a large SOMO-SOMO overlap and high molecular symmetry, which will
enhance the electron and hole mobilities and may serve as potential building blocks
to construct thermoresponsive and photoresponsive conductive and magnetic
materials [
4
,
58
]. But above all, the stability issue has to be carefully examined.
The first attempt to synthesize triangulene was made by Clar, but only
polymerized product was obtained due to its kinetic instability [
59
]. In 1977, a
closed-shell dianion of triangulene was achieved and detected by NMR
measurements [
60
]. In 2001, Nakasuji et al. applied the same strategy that stabilizes
the phenalenyl radical to the triangulene system, by introducing bulky
tert
-butyl
groups on three vertexes of the triangle, with the aim of increasing kinetic stability
and minimizing the electronic perturbation. The synthetic route was shown
in Scheme
4
and the biradical
p
species was generated by treating the