Chemistry Reference
In-Depth Information
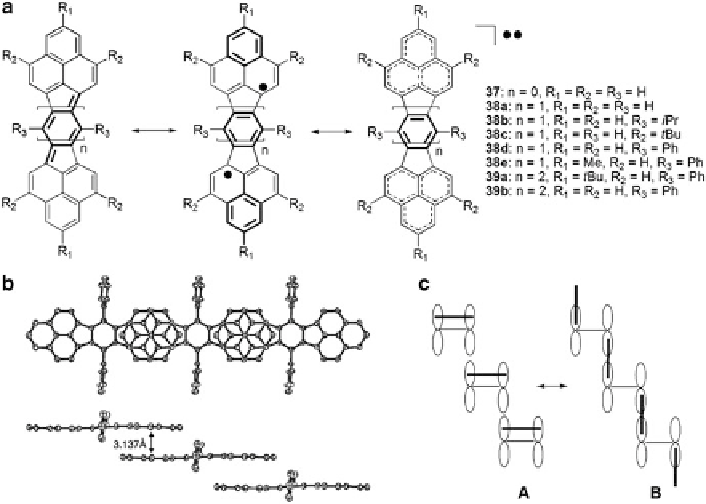
Fig. 11 (a) Resonance forms of bis(phenalenyls) with different aromatic linkers; (b) crystal
structure of 38d [
37
]; (c) the RVB model for the electronic structure of 38e [
38
]
in the NMR spectra at elevated temperatures as well as line sharpening at lower
temperatures indicated a thermally accessible triplet species, and the small singlet-
triplet energy gap can be determined by solid state ESR and SQUID measurements.
One of the most salient features derived from a profound biradical character was the
strong intermolecular interactions in the solid state. In 2005, Kubo reported a phenyl-
substituted IDPL 38d and its packing motif in the solid state, initiating a hot
discussion on the interacting motif between and within those systems. The crystal
structure of 38d demonstrated one-dimensional chains in staggered stacking mode
with an average
distance of 3.137
´
, which is significantly shorter than the van
der Waals contact of carbon atoms (3.4
´
)(Fig.
11b
). This packing mode will
maximize the SOMO-SOMO overlapping between the radicals, leading to stabilized
intermolecular orbitals that correspond to intermolecular covalency [
37
]. Further
evidence of the coexistence of intermolecular and intramolecular interactions was
provided by Huang from a theoretical perspective [
42
]. They found that the partici-
pation of unpaired electrons in intermolecular
p
-
p
bonding made them partially
localized on phenalenyl units but less available for intramolecular delocalization,
i.e., the intermolecular interaction is more predominant. With the aim of better
understanding the intermolecular and intramolecular spin-spin interactions, Shimizu
et al. attempted to alter the magnitude of the interactions by varying the external
conditions such as molecular structure, temperature, and pressure. Interestingly, they
found a larger intermolecular separation (3.225
´
) when introducing a methyl group
p
-
p