Chemistry Reference
In-Depth Information
Pd/C
31
7
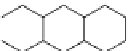
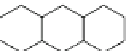
Fig. 12 Catalytic dehydrogenation of hexacosahydroheptacene 31 was claimed to yield heptacene
[
15
]
Another synthesis of a heptacene was reported by Bailey and Liao in 1955
[
15
]. These authors obtained heptacene in 76% yield by dehydrogenation of a
hexacosahydroheptacene 31 with palladium-on-carbon at 340-375
C (Fig.
12
).
Bailey and Liao give a satisfactory elemental analysis and describe their product
as greenish-black material that decomposes at approximately 400
C[
15
]. This
work received criticism by Boggiano and Clar [
42
]. No further attempts at
heptacene synthesis were described in the twentieth century, with the exception
of a Ph.D. thesis [
47
].
A breakthrough was achieved by Neckers and co-workers who reported
the first unequivocal synthesis of heptacene in 2006 [
48
]. This group used
the Strating-Zwanenburg [
49
] reaction, i.e., the photobisdecarbonylation of the
bridged
-diketone 37 [
50
,
51
], in a PMMA matrix (Fig.
13
)[
48
]. The heptacene
photoprecursor 37 is accessible from 2,3-dibromonaphthalene 32 and 5,6,7,8-
tetramethylidene-bicyclo[2.2.2]oct-2-ene 33. First, the aryne generated from 32
undergoes Diels-Alder reactions with 33 and furnishes the hydrocarbon 34. Aro-
matization of 34 to 35 is followed by oxidation of the etheno bridge. This was
achieved by the osmium tetroxide catalyzed dihydroxylation of 35 and 36 and
subsequent Swern oxidation.
Heptacene 7 was characterized by its UV/vis spectrum that shows the typical
vibrational progression in the region of the
p
band (
α
λ
max
760 nm). Under ambient
conditions, the compound isolated in the PMMA matrix disappears within 4 h
presumably due to reaction with atmospheric oxygen molecules that diffuse into
the matrix [
48
]. The persistence is less than that of hexacene under similar
conditions. Note that photogeneration of heptacene in solution was not possible.
In oxygen saturated solutions, oxygenated products rather than the hydrocarbons
were obtained by Mondal et al. [
48
] according to MALDI-MS and
1
H NMR
investigations.
Further matrix isolation experiments were reported by Bettinger et al. using solid
noble gas matrices (Ar and Xe) under cryogenic conditions [
22
,
52
]. Heptacene was
generated photochemically as reported by the Neckers group [
48
]. These
experiments allowed measurement of the UV/vis absorption spectrum of heptacene
between 200 and 800 nm (Fig.
14
) and its IR spectrum (Fig.
15
).
It was also observed that the acenes (including pentacene and hexacene) can
undergo a photochemically (high energy line of a low pressure mercury lamp)
initiated redox process that generates acene radical cations and radical anions
within the same matrix (Fig.
16
)[
22
,
52
]. Assignment of the electronic transitions
to the positively charged radical was possible by doping the noble gas matrix with
better electron acceptors such as dichloromethane. This results in the suppression of