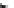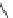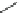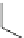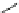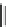Chemistry Reference
In-Depth Information
O
O
NaOH
Zn
HOOC
COOH
HOOC
COOH
28
NaCl
ZnCl
2
NaCl
ZnCl
2
andother isomers
29
andother isomers
as well as tetrahydroheptacenes
30
-H
2
Dehydrogenation
Cu, 320-330°,
1 mm CO
2
7
30
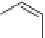
Fig. 11 Synthesis of heptacene 7 or 1,2-benzohexacene 30 [
41
,
46
]
derivative 29) or tetrahydroheptacenes using copper bronze and a CO
2
stream at
310-320
C (Fig.
11
). Clar describes that heptacene is extremely reactive and
difficult to obtain in a pure state. He repeated the latter statement in some of his
subsequent papers [
42
,
43
]. Nonetheless, he describes heptacene as an almost black,
grass green as a thin film, compound. It is said to be very poorly soluble in boiling
1-methylnaphthalene or phenanthrene with a green color. These solutions immedi-
ately decolorize upon contact with air or maleic anhydride. Clar observed bands in
1-methylnaphthalene solution in the visible at 736, 657, 594, 520, and 503 nm.
He assigned the first three bands to the
p
band system and the last two to the
band
system. Based on calculations, he predicted the longest wavelength absorption at
around 840 nm and the
ß
band at 381 nm in this solvent.
With regard to the identity of the dihydroheptacenes there remains some ambi-
guity, as in the following year, 1943, Charles Marschalk [
44
] reiterated [
45
] that the
orange-brown compound he had ascribed previously to 6,17-dihydroheptacene 29
does not isomerize to 7,16-dihydroheptacene, in contrast to Clar's [
41
] report.
It also does not from heptacene under dehydrogenation conditions. However, he
confirmed that among the hydrocarbon mixture Clar obtained from reductive
cyclization of 28 there was an orange-brown compound that gave a highly reactive
green hydrocarbon upon dehydrogenation with Pd/C in trichlorobenzene [
44
]. Its
absorption spectrum agreed with that assigned by Clar [
41
] to heptacene [
44
].
Clar and Marschalk summarized their efforts towards heptacene in a joint paper in
1950 [
46
]. Dihydroheptacenes are confirmed not to give heptacene upon attempted
dehydrogenation by copper or Pd/C. On the basis of similar UV/vis spectra the highly
reactive green compound was assigned to 1,2-benzohexacene 30.Theauthors
suggested that reductive cyclization of 28 may have resulted in angularly annulated
dihydrobenzohexacenes, such as 30-H
2
displayed in Fig.
11
[
46
].
ʱ