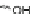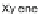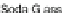Chemistry Reference
In-Depth Information
Scheme 42 Preparation of parent [2,1-
c
]IF dione 149 [
91
]
6
Indeno[2,1-
c
]fluorenes
6.1
Indeno[2,1-
c
]fluorene-5,8-diones
The first account of the [2,1-
c
]IF dione in the literature occurred in 1961 where
work by Ginsburg and Altman investigated the Diels-Alder chemistry regarding bi
(cyclopentenes) and bi(cycloheptenes) (Scheme
42
)[
91
]. They found that reaction
of indanone 143 in the presence of Al amalgam resulted in pinacol 144, which was
further eliminated in refluxing acetic acid and acetic anhydride to form 3,3
0
-
biindenyl 145. Cyclization of 145 with maleic anhydride (146) afforded anhydride
147. Decarboxylation of 147 using elemental Cu, barium hydroxide, and soda glass
at 400
C generated 5,8-dihydroindeno[2,1-
c
]fluorene 148; subsequent oxidation
with sodium dichromate in refluxing acetic acid afforded dione 149.
An alternative route to 149 was devised by Chardonnens and Chardonnens in
1966 where propiophenone (150) is converted to pinacol 151 through an aluminum
amalgam in refluxing ethyl acetate (Scheme
43
)[
92
]. Double elimination of 151
using acetyl chloride in refluxing acetic anhydride afforded diene 152 which is
cyclized with 146 to give anhydride 153. Aromatization of the central ring using
elemental sulfur at 280
C formed terphenyl anhydride 154. Saponification to 155
and subsequent decarboxylation using the Lazier catalyst at 215
C yielded 3,6-
dimethyl-1,2-diphenyl benzene (156). Oxidation via potassium permanganate in a
refluxing solution of water and pyridine formed diacid 157 and further cyclization
using concentrated sulfuric acid at elevated temperatures gave 149.
The few examples of substituted [2,1-
c
]IF diones in the literature are limited
to the 1-, 6-, 7-, and 12-positions. For example, work by Wong and coworkers
on the investigation of planarized cyclooctatetraenes cyclized diacid 158,
with polyphosphoric acid at elevated temperatures to form dione 159 where the
1- and 12-positions are tethered together with an ethane bridge (Scheme
44
)[
93
].