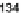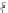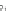Chemistry Reference
In-Depth Information
Fig. 16 Resonance structures of 4
Scheme 39 Preparation of fully-conjugated 11,12-dimesitylindeno[2,1-
b
]fluorene 134 (Shimizu
and Tobe, 2011, private communication)
5.2 Fully-Conjugated Indeno[2,1-
b
]fluorenes
Full conjugation for the indeno[2,1-
b
]fluorene isomer is also possible (4, Fig.
16
).
However, due to ring positioning, formation of the requisite xylylene core in 4
requires the disruption of two benzene rings rather than one needed for the [1,2-
b
]
and [2,1-
a
] isomers. As such, 4 should display an asymmetrical arrangement of the
p
-system where the D- and E-rings both possess
s
-
cis
diene linkages. Like the
above-mentioned fully conjugated [2,1-
a
] 3, interest for 4 lies in its ground state
biradical character (4b).
Currently, only one example of a fully-conjugated indeno[2,1-
b
]fluorene is
known and its existence at this time remains unofficial (Shimizu and Tobe, 2011,
private communication). Tobe et al. synthesized the molecule in a near identical
manner in which 115 was made - IF dione 17 was reacted with 2 equiv.
mesitylmagnesium bromide to form diastereomeric diols 133. Further reaction
with SnCl
2
in the presence of trifluoroacetic acid yielded 10,12-dimesitylindeno
[2,1-
b
]fluorene 134 (Scheme
39
). Little is known about this molecule in terms of
optoelectronic or structural properties. Due to the presence of the two
s
-
cis
diene
linkages, however, 134 is considerably less stable than its [1,2-
b
] and [1,2-
a
]
counterparts.
5.3 Other Indeno[2,1-
b
]fluorenes
In addition to the spiro-fused [1,2-
b
]IF and [2,1-
a
]IF, Rault-Berthelot and
colleagues also synthesized [2,1-
b
] derivative 137 for use in OLED devices
(Scheme
40
). They found that the compound had one of the highest triplet energies