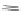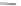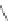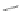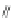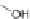Chemistry Reference
In-Depth Information
Scheme 37 Original
preparation of parent indeno
[2,1-
b
]fluorene dione 17 [
26
]
Scheme 38 Chardonnen's
route to 17 [
27
]
5
Indeno[2,1-
b
]fluorenes
5.1
Indeno[2,1-
b
]fluorene-10,12-diones
The first account of 10,12-indeno[2,1-
b
]fluorene dione 17 dates to 1951 when
Deuschel condensed phenylbenzoylacetylene (125) with ketodiester 126 to afford
m
-terphenyl diester 127 (Scheme
37
)[
26
]. Saponification of 127 to 128 and
subsequent ring closure yielded 11-hydroxy-[2,1-
b
]IF dione 129. Reduction using
Zn metal provided 10,12-dihydro [2,1-
b
]IF 100. A final oxidation with sodium
dichromate gave dione 17.
Chardonnens and Ritter in 1955 reported an alternative pathway to 17 where 4,6-
dibromoisophthalic acid (130) was converted to the acid chloride and subsequently
underwent a twofold Friedel-Crafts acylation with benzene to afford dibromodione
131 (Scheme
38
)[
27
]. Aryl amination of 131 yielded 132 where a subsequent
Sandmeyer-mediated cyclization gave 17 in 79% yield. The synthesis of substituted
indeno[2,1-
b
]fluorenes has also been explored. As discussed earlier, the [2,1-
b
]isomer
was often formed in equal amounts with the corresponding [1,2-
a
]isomerina
multitude of cyclizations performed by Chardonnens and coworkers (Scheme
3
)[
43
].