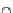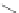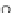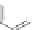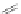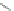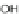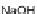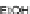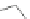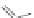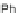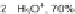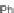Chemistry Reference
In-Depth Information
Scheme 34 Diels-Alder approach to the preparation of 115 [
33
]
Fig. 14 Resonance structures of 3
Scheme 35 Preparation of fully conjugated 11,12-diphenylindeno[2,1-
a
]fluorene 123a [
88
]
4.2 Fully-Conjugated Indeno[2,1-
a
]fluorenes
Like 2, the indeno[2,1-
a
]fluorene isomer also supports full conjugation, illustrated
by 3, where an
o
-xylylene core (
o
-quinodimethane) is present in the structure
(Fig.
14
). As such, interest in this structure lies in the potential of the
o
-xylylene
core to exist as a stable open shell configuration (3b).
Work on 3 originated in 1957 when LeBerre reacted phenylmagnesium bromide
with 115 to form diol 121 (Scheme
35
)[
88
]. Refluxing in a hydrobromic acid/acetic
acid solution resulted in dibromide 122, where reduction using elemental Cu
afforded diphenylindeno[2,1-
a
]fluorene 123a in 63% yield. While 123a readily
degraded in aerobic conditions, solutions under an inert atmosphere were said to
be stable but no information regarding time was given. However, due to these air-
sensitivity issues, no further investigation of its structural or electronic properties
was explored.
Recent work by Tobe examined the synthesis of 11,12-dimesitylindeno[2,1-
a
]
fluorene 123b, which was also derived from 115 (Scheme
36
)[
89
]. After reaction of
115 with mesitylmagnesium bromide, diol 124 was reduced with anhydrous SnCl
2
in the presence of trifluoroacetic acid at elevated temperatures in toluene to afford
123b in 48% yield. Fortunately, 123b was stable at least for 1 week in light and air
and showed no reactivity with maleic anhydride. The researchers attributed this