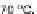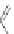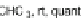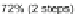Chemistry Reference
In-Depth Information
Scheme 32 Synthesis of 6,12-tetrafluoro [1,2-
b
]IFs 112a-g [
87
]
Scheme 33 Preparation of parent indeno[2,1-
a
]fluorene dione 115 [
23
,
26
]
4
Indeno[2,1-
a
]fluorenes
4.1
Indeno[2,1-
a
]fluorene-11,12-diones
The first account of the indeno[2,1-
a
]fluorene core in the literature dates to 1939
when Weizmann investigated polycyclic structures and their potential carcinogenic
properties [
23
]. Intramolecular Friedel-Crafts acylation of 3,6-diphenylphthalic
anhydride (113) in refluxing CS
2
afforded fluorenone carboxylic acid 114
(Scheme
33
). Acid chloride formation and subsequent cyclization in concentrated
sulfuric acid generated the parent indeno[2,1-
a
]fluorene dione 115 in 5% yield with
respect to 113. This was reaffirmed by Deuschel in 1951 where 115 is obtained in
72% under the same conditions [
26
].
Recent work by Rault-Berthelot et al. considered an alternative pathway to 115
(Scheme
34
)
33
]. Diels-Alder cyclization with
trans
,
trans
-1,4-diphenyl-
1,3-butadiene (116) and dimethyl acetylenedicarboxylate (117) resulted in
cyclohexadiene 118 where subsequent oxidation with Pd/C afforded terphenyl
119. Direct cyclization of 119 using concentrated sulfuric acid does afford 115,
but only in 12% yield. Instead, saponification of 119 to diacid 120 followed by
cyclization improves the yield of 115 to 38%.