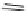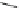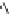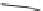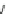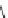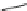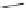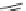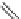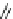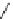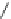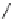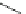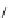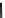Chemistry Reference
In-Depth Information
R
3
Si
R
3
Si
R
3
Si
SiR
3
R
3
Si
SiR
3
SiR
3
SiR
3
25
24
SiR
3
SiR
3
SiR
3
SiR
3
R
3
Si
R
3
Si
O
O
SiR
3
SiR
3
27
26
Fig. 10 Dimers of TIBS-hexacene.
Top left
: product 24 obtained under laboratory light;
top right
:
product 25 obtained under air in the dark;
bottom left and right
: Diels-Alder product 26 and its
endoperoxide 27 [
33
]
present in 26, the compound is green but turns yellow during column chromatogra-
phy. This yellow product could be identified as an endoperoxide 27 by single-
crystal X-ray crystallography [
33
].
The major pathway of decomposition of the silylethynyl substituted hexacenes is
thus dimerization either in the light or in the dark. Endoperoxide formation is a
mode of degradation only if one of the protecting ethynyl groups is compromised.
Very recently Anthony and co-workers reported the synthesis of the 1,2,3,4-
tetrafluoro as well as the 1,2,3,4,9,10,11,12-octafluoro derivatives of 23e and the
characterization of their charge transport properties [
39
]. The octafluoro compound
was also investigated as an electron acceptor in organic bulk heterojunctions [
40
].
3 Heptacenes
3.1 Parent Heptacene
In 1942 Clar reported the synthesis of heptacene [
41
]. As with hexacene, the key to
the synthesis is the dehydrogenation of dihydroheptacenes (such as the 6,17-dihydro