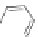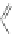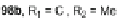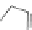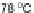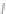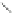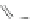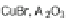Chemistry Reference
In-Depth Information
Scheme 28 Synthesis of 26 and [2,1-
b
]IF 100 [
79
]
Scheme 29 Synthesis of polymer 103 starting from 26 [
80
]
insertion of the metal leads to intramolecular aryl-aryl couplings via subsequent
reductive eliminations from the palladium center. This elegant reaction can produce
both 6,12-dihydro[1,2-
b
]IFs and 10,12-dihydro[2,1-
b
]IFs in greater than 90% yield
from the appropriate terphenyl precursor.
Many synthetic routes are utilized to generate 6,12-functionalized
dihydroindeno[1,2-
b
]fluorenes, typically from 22 or 26. One direct method is
base-promoted alkylation of the methylene carbons of 26. For example, lithiation
of 26 followed by treatment with 1-bromooctane gave tetrasubstituted 101
(Scheme
29
)[
80
]. Subsequent bromination at the 2- and 8-positions with CuBr/
alumina furnished 102, which could then be polymerized to form polyindeno-
fluorene 103 linked at the 2- and 8-positions rather than at the 6- and 12-positions
as seen in Scherf's polyindenofluorene 85. Muellen and co-workers found that
103 showed a bathochromic shift in fluorescence compared to polyfluorene (into
the visible region) and it formed a liquid crystalline phase at high temperature,
possibly making a suitable LED component.
An alternative route to functionalized dihydroindeno[1,2-
b
]fluorenes begins
from fluorenone and involves intramolecular Friedel-Crafts alkylation in the final
step, leading to spiro-fused derivatives - a method championed by Rault-Berthelot,