Chemistry Reference
In-Depth Information
R
3
Si
O
OH
1. R
3
SiCCLi
2. sat. NH
4
C
l
OH
21
22
O
SiR
3
R
3
Si
SnCl
2
/10% HCl
23
a: R =
i
-Pr
b:R=
t
-Bu
c: R=
i
-Bu
d:R=Cyclopentyl
e: R = Cyclohexyl
f: R=SiMe
3
SiR
3
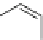
Fig. 9 Synthesis of silylethynyl substituted hexacenes 23 [
37
]
chemistry [
35
,
36
], and the group of Anthony has demonstrated that this strategy
can result in an increased stability of the higher acenes.
In 2005, Payne et al. reported the first synthesis of a functionalized hexacene
starting from quinone 21 (Fig.
9
)[
37
]. The tri-
iso
-propylsilyl group (23a) is too
small to stabilize the hexacene in solution or in the solid state. The bulkier tri-
tert
-
butylsilyl (TTBS) group, however, provided sufficient stabilization to allow com-
plete spectroscopic characterization of the corresponding hexacene derivative.
Single crystal X-ray crystallography demonstrated that the acene unit is planar to
within 0.1
˚
and the alkyne groups are bent. This was ascribed to packing effects
[
37
]. In spite of the stabilizing silylethynyl groups, the hexacene slowly
decomposes in solution in the presence of air and light. The crystals, on the other
hand, can be stored for several months in the dark [
37
].
The decomposition of the hexacenes was followed up by the Anthony group
[
33
]. Particularly interesting results were obtained with the tri-
iso
-butylsilyl (TIBS)
group that is smaller than the TTBS group investigated earlier. Under laboratory
light, TIBS-hexacene was found to form a centrosymmetric dimer 24
interconnected between the C7 and C14 positions of both TIBS-hexacenes
(Fig.
10
)[
33
]. On the other hand, pure crystals of TIBS-hexacene kept under air
in the dark produced a different dimer 25 with new bonds between the C7/C14
positions of one hexacene unit and the C8/C13 positions of the other hexacene
molecule (Fig.
10
)[
33
].
The centrosymmetric dimer 24 formed by reaction between the two most
reactive sites (C7 and C14) and in this sense resembles the photodimers of parent
anthracene, tetracene, and pentacene [
38
]. The dimer 25 formed in the dark reaction
resulted from reaction of the most reactive ring of one monomer with the next to
terminal ring of the other molecule [
33
]. Another interesting dimer 26 was isolated
as a by-product of the hexacene synthesis by the Anthony group [
33
]. It is formed
by Diels-Alder reaction between the reactive ring containing the C7 and C14 atoms
and the alkynyl group of another TIBS-hexacene. As one intact hexacene unit is