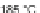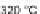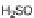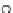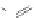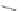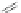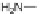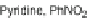Chemistry Reference
In-Depth Information
Scheme 5 Chlorination/
oxidation route to 22 [
26
]
Scheme 6 Na/NH
3
-induced transannular cyclization of 24 [
41
]
Scheme 7 Nitration, reduction, and amidation of 22 [
42
]
sodium sulfide afforded diaminodione 30 in 95% yield, which in turn was amenable
to amidation in a pyridine/nitromethane solution at 180
C to give 31. While the
sites for nitration of 22 were most likely on the peripheral rings, specific informa-
tion about their exact positions remains unknown.
Chardonnens and Salamin also synthesized a number of substituted [1,2-
b
]IF
diones, which were typically made in the same fashion as the [1,2-
a
]IF diones
described earlier (Scheme
3
)[
43
]. For example, treatment of 2,5-dibromoterephthalic
acid (32) with thionyl chloride and subsequent Friedel-Crafts acylation with 33a-d
provided 34a-d (Scheme
8
). Intramolecular arylation at elevated temperatures
furnished IF-diones 35a-d in 20-28%yield. This technique has also been successfully
applied to asymmetric 1,3,4-trisubstituted diones as well as 2,3,8,9-tetrasubstituted
diones [
42
].