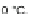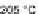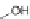Chemistry Reference
In-Depth Information
Scheme 4 Synthesis of parent [1,2-
b
]IF dione 22 [
26
]
fluorenone (15) also formed in equal yield as addition could occur at either the 6- or
8-positions of the fluorene unit. Addition of either indenonyl ketone 16a or 16b,
respectively, under similar conditions afforded both 12 and parent [2,1-
b
]IF dione 17
in 28% and 31% yield, respectively. Furthermore, oxidation of 14 and 15 using
sodium dichromate at elevated temperatures led to the expected diones 12 and 17.
Despite the formation of two discrete condensation products, both 14 and 15 as well
as 12 and 17 can be isolated separately either through soxhlet extraction or by
selective crystallization. Both techniques take advantage of the key topological
differences that the [1,2-
a
] and [2,1-
b
] structures possess - namely, the [2,1-
b
]
isomer is more soluble than the [1,2-
a
] isomer. The greater dipole moment induced
by the
syn
arrangement of the ketones makes the [2,1-
b
]IF scaffold more amenable
to dissolution in polar solvents, allowing for easy separation of the isomers.
The first account of the [1,2-
b
]IF skeleton dates to 1951 when Deuschel
alkylated
p
-xylene (18) with 2 equiv. cyclohexene to give tricycle 19 (Scheme
4
).
Treatment with Pd/C afforded 1,4-dimethyl-2,5-diphenylbenzene (20). Oxidation
of the methyl moieties using potassium permanganate and pyridine generated
diacid 21. Subsequent cyclization using concentrated sulfuric acid gave the parent
[1,2-
b
]IF-6,12-dione 22 in 87% yield [
26
].
Alternatively, passing a stream of chlorine gas in a neat solution of 20 at 185
C
afforded 23 in 46% yield (Scheme
5
). A refluxing aqueous solution of CuCl
2
also
oxidized 23 to dione 22.
Access to the [1,2-
b
]IF core was also discovered through collapse of the
dehydrobenzo[12]annulene scaffold. First described by Eglinton and coworkers in
1960 (Scheme
6
), tetrayne 24 underwent a double transannular cyclization when
reacted with elemental Na in liquid ammonia to provide octahydroindeno[1,2-
b
]
fluorene 25 as a mixture of two isomers in 69% yield [
41
]. Further treatment
with 15% Pd/C aromatized the central ring to afford 6,12-dihydroindeno[1,2-
b
]
fluorene (26).
Continued work by Deuschel demonstrated that 22 was amenable to nitration
using potassium nitrate in concentrated sulfuric acid at 20
C (Scheme
7
)[
42
]. By
varying the amount of electrophile used, [1,2-
b
]IF diones could be nitrated either
two (27), three (28), or four (29) times. Reduction of dinitro 27 with aqueous