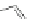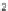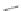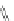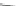Chemistry Reference
In-Depth Information
Fig. 1 Nomenclature and
numbering for indenofluorene
isomers.
Italicized
numbers
denote numbering for
methylene bridge orientation,
non-italicized
numbers
denote atom
were a sporadic topic in the literature for roughly 70 years [
17
-
24
] until the
pioneering work by Deuschel [
25
,
26
] and Chardonnens [
27
] in the 1950s devised
a general strategy for their synthesis. However, at that time modern spectroscopic
and structural identification techniques were rare in the scientific community; thus,
IFs were not typically characterized beyond their melting points and elemental
analyses. Outside their initial syntheses, IF development languished for another
40 years. A decade ago, following the realization of CP-PAHs as viable organic
materials, interest in the IF scaffold resumed. Since then derivatized IFs have been
recognized as potential candidates for stable emissive materials [
28
-
34
] and more
recently identified as a semiconducting material in devices [
35
-
37
].
The indenofluorene family is comprised of five structural isomers which can be
difficult to discern as multiple naming and numbering strategies as well as graphical
presentations have been employed over the last century to describe their shape and
structure (Fig.
1
). Current nomenclature uses a bracketed set of numbers and a letter
to distinguish one isomer from another where the set of numbers refer to the
orientation of how the indene group faces the fluorene and the letter corresponds
to the edge of the indene/fluorene ring fusion. Both “1,2” isomers exhibit an
anti
relationship between the methylene bridges of the five-membered rings, while the
three remaining “2,1” isomers exhibit a
syn
relationship. The IF scaffold exhibits a
variety of symmetries: the [1,2-
a
] isomer is only centrosymmetric, the [1,2-
b
]
isomer exhibits rotational symmetry, and the [2,1-
a
], [2,1-
b
], and [2,1-
c
] isomers
possess a mirror plane. As such, each isomer has its own unique ring topology,
which in turn has pronounced structural and electronic consequences.
This review will cover the five IF regioisomers and describe common synthetic
procedures used to obtain each form, including assorted structural, optoelectronic,
and materials properties as available. IFs possessing sp
3
-hybridization at the
bridgehead of the five-membered rings is a burgeoning area of research in the
realm of emissive materials worthy of its own review; however, only salient
examples will be described. Device information will be presented assuming the
reader has a basic optical and electronic materials background. Greater detail can be
found in the appropriate references.