Chemistry Reference
In-Depth Information
a
300 K
600 K
b
450 K
525 K
two-dimensional graphite?
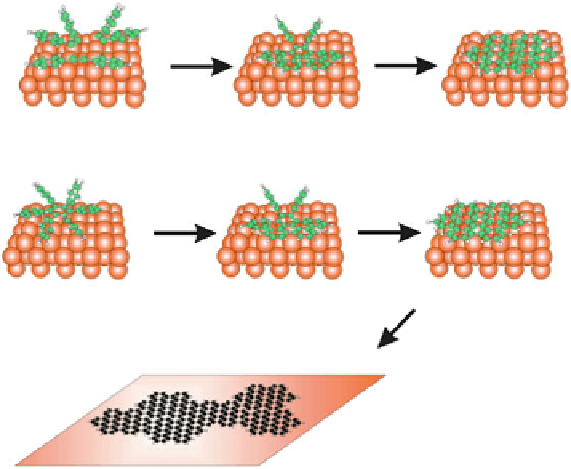
Scheme 47 Proposed schemes for the thermally induced reactions of (a) diphenylacetylene and
(b) hexaphenylbenzene on Cu(110) [
115
]
on the surface to form extended graphene ribbons. Depending on the monomers
employed, different geometries of the final structures can be realized (Scheme
48
).
A similar approach has also been put forward by the group of Hecht by
presenting a suitable monomer to accomplish the on-surface polymerization [
118
].
Alternatively, small PAHs can be self-assembled on a surface and then
connected via dehydrogenation to form high-quality large-area graphene. However,
there is no direct control of the exact size and periphery of the prepared
material [
119
].
5 Summary, Conclusions, and Outlook
The long history in organic chemistry of PAHs has been a solid basis for a
continuous development of methods to access large graphene-type structures. The
main drawback of applying organic synthetic methods towards the preparation of
graphene consists in the low solubility of such structures in common organic
solvents. Nevertheless, powerful strategies have been put forward in recent years,
also combining classical approaches with new ideas, such as on-surface reactions.
Although the area is still in its infancy, not all efforts could be covered and a
selection has been made to illustrate the progress and the potential of the current
state-of-the-art in organic chemistry. The future will further enhance the
possibilities to create new large PAHs with defined structures exhibiting the
exciting properties of graphene. With a reliable method in hand to access