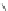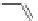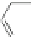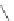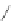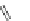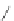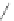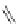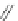Chemistry Reference
In-Depth Information
O
O
H
cross-
coupling
70-9%
O
H
H
TsNHNH
2
B(OH)
2
X
145
146
O
H
147
R
1
R
4
H
NNHTs
[Rh
2
(OAc)
4
] ) (1.5 mol %)
LiO
t
Bu (3 equiv)
4Å MS, toluene
90 ºC, 1 h
R
2
R
3
TsHNN
H
149
148
92%
Scheme 37 Formation of phenanthrene scaffold 149 by a Rh-catalyzed cyclization of bis
(
N
-tosylhydrazone)s 148 (X
¼
I, Br, Cl, OTf) [
93
]
1)
n
BuLi, THF -78 ºC
2) Cr
Cl
3
, rt
3)
Br
Ph
Ph
48%
Br
150
151
Scheme 38 Cr-mediated synthesis of PAHs [
94
]
Pd(OAc)
2
(5 mol %)
TlOAc (1 equiv)
CsF (3 equiv)
MeCN/toluene, 90 ºC 8 h
Ph
I
TMS
EtO
2
C
OTf
Ph
85%
152
153
EtO
2
C
154
Scheme 39 Pd-catalyzed cyclization of an aryliodide, alkyne and an in situ formed aryne [
96
]
R
Ph
R
Pd(OAc)
2
(7.5 mol %)
AgOAc (2 equiv)
CH
3
CN, 110 ºC, 36h
R = Me 60%
R = H 17%
H
Ph
Ph
H
R
Ph
R
155
156
Scheme 40 Pd-catalyzed annulation of unfunctionalized aromatics [
97
]
A Rh-based strategy was also put forward involving aryl boronic acids and air as
the oxidizing agent with Cu as a co-catalyst for the oxidation (Scheme
41
)[
98
].
Electron-rich as well as electron-poor boronic acids are well tolerated.