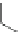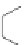Chemistry Reference
In-Depth Information
R
R
R
I
I
I
AuCl (20 mol %)
toluene, 60 ºC, 24h
I
I
I
R
R
R
141
140
139
3% (NMR)
97% (NMR)
1)
n
-BuLi
2) H
2
O
R
R
R
R
142
143
Scheme 35 Au-catalyzed cyclization to access dibenzo[
a
,
h
]anthracene (
R ¼ t
-Bu; no yield for
the second step is given) [
91
]
TpRuPPh
3
(CH
3
CN)
2
PF
6
(5 mol %)
DCM, 80 ºC, 36 h
16
144
86%
Scheme 36 Synthesis of coronene via four-fold Ru-catalyzed cyclization [
19
]
To increase further the variability, methods starting from three or more reaction
partners have been developed. Iodobenzene derivatives can be combined with an
alkyne as well as an in situ formed aryne species under Pd-catalysis to efficiently
form the phenanthrene core 154 (Scheme
39
)[
96
].
The group of Wu showed that it is possible to perform a benzannulation on an
unfunctionalized aromatic compound using two alkynes and a Pd catalyst
(Scheme
40
)[
97
]. The presence of electron-releasing groups increases the yield.
If benzene is used as the starting material, only a low yield could be obtained. Two
different mechanistic proposals are discussed, either involving a palladacyclo-
pentadiene or participation of an Ar-Pd species in the first step of the cycle.
AgOAc functions as the oxidizing agent to regenerate the Pd(II) species.