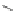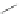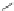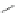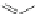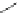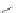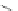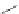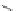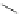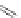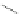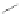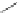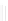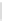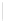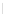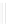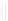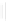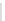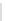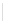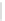Chemistry Reference
In-Depth Information
O
O
t
Bu
t
Bu
t
Bu
t
Bu
Cl
1)
n
BuLi, tetrachloro-
bianthrone
2) SnCl
2
,AcOH
OMe
t
Bu
t
Bu
KOH,
quinolone
Cl
Cl
58%
36%
Br
128
Cl
t
Bu
t
Bu
t
Bu
t
Bu
O
O
130
129
1) ArMgBr, CeCl
3
2) SnCl
2
,AcOH
62%
Ar
Ar
t
Bu
t
Bu
t
Bu
t
Bu
Ar =
DDQ,
Sc(OTf)
3
43%
t
Bu
t
Bu
t
Bu
t
Bu
Ar
Ar
132
131
Scheme 32 Synthesis of quateranthene 132 [
82
]
2.5 Annulation Reactions
The extension of aromatic systems by annulation reactions is an effective way of
extending smaller PAHs into larger graphene-like structures [
83
]. In this context
multiple approaches have been devised.
2.5.1 Photocyclizations
Classical methods rely on electrocyclization reactions promoted by heat or, espe-
cially, by photochemical means, usually followed by an oxidation to deliver the
aromatic compound. One of the most prominent routes is also known as the Mallory
reaction, first reported in 1964 [
84
,
85
]. Since then it has been further optimized
[
86
] and applied, especially in the synthesis of helicenes [
87
], but also in a number
of syntheses of graphene-type structures. Zhang et al. recently combined the
Mallory reaction with a Scholl cyclization to access tetrabenzocoronene 135
(Scheme
33
)[
88
]. A similar approach has been reported for the preparation of
hexabenzocoronene [
62
].