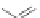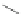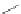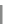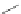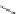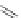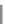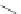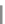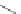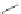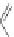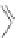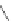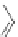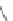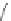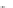Chemistry Reference
In-Depth Information
16
123
124
125
126
127
Scheme 31 Selected products of the complex mixture of the intermolecular Scholl reaction of
coronene 16 [
81
]
The more common way to connect two unfunctionalized aromatic rings is via the
Scholl reaction, which has been employed early on in the synthesis of PAHs and has
already been mentioned several times in this chapter [
78
]. In this case, a metal salt,
such as Fe(III)Cl
3
[
79
] or Al(III)Cl
3
, is employed which acts as the oxidizing agent.
Examples shown earlier by the M
¨
llen group feature this synthetic tool extensively,
and it has proven its applicability to very large nano-graphene structures. Although
very efficient in intramolecular coupling reactions, the Scholl reaction is less
selective in intermolecular variations, giving rise to complex product mixtures
[
80
]. In the example shown in Scheme
31
, coronene 16 has been subjected to
Scholl reaction conditions. Besides the dimers 123 and different isomers of trimer
of coronene 125-127, five-membered ring fusions have also been observed. A
similar outcome has been observed by simply heating coronene to 550-600
C[
81
].
A nice feature of reductive as well as oxidative coupling reactions has been
presented recently in the synthesis of quateranthene 132 (Scheme
32
)[
82
]. After
addition of the lithiated species of 128 into the bianthrone and dehydrogenation,
base induced cyclization gave the intermediate 130. Grignard addition to the ketone
and subsequent reduction delivers 131, which was then fully aromatized by a
Scholl-reaction involving DDQ as an oxidant in the presence of Sc(OTf)
3
.