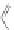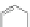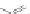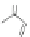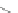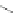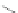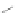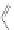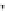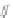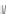Chemistry Reference
In-Depth Information
TIPS
TIPS
57
TIPS
61
O
TIPS
1) 200°C, 11d, 72%
2)TBAF, 89%
O
190°C
66%
60
TIPS
58
TIPS
59
62
1)190°C,89%
2)Cu(OTf)
2
/AlCl
3
, 62%
1)Co
2
(CO)
8
, 100°C, 71%
2)Cu(OTf)
2
/AlCl
3
, 62%
O
60
63
Scheme 16 Synthesis of a giant 222 graphite sheet 63 [
41
]
Diels-Alder reactions. Bulky protecting group were used on the outer alkynes,
encouraging a selective Diels-Alder reaction. However, the steric bulk slowed
down the reaction rate, which required 11 days for complete conversion. The
obtained polyphenylene was then planarized by cyclodehydrogenation to give the
hexagonal graphene sheet 63 which was characterized by MALDI spectrometry
(Scheme
16
).
There are two main edge formations possible in graphene type structures as
depicted in Fig.
3
. It has been shown that the periphery of graphene plays a crucial
role in its electronic structure, self-assembly behavior, and reactivity. Zig-zag and
armchair edges also provide different reactive sites for further functionalization.
In the following, examples featuring the Diels-Alder/dehydrogenation strategy
are presented. By choosing substituted cyclopentadienones and substituted alkynes,
a myriad of graphene-type structures of different architectures varying in sizes and
periphery can be accessed [
42
-
44
]. Thus, by modifying the cyclopentadienone 64