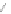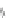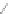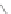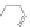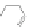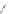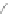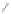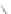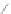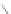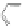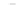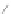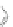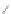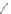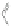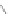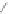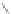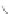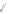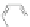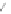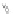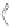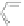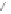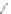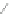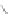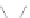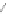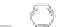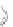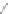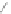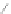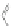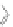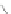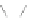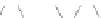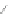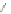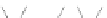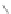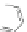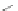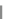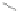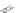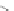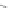Chemistry Reference
In-Depth Information
R
R
R'
R'
R
R
39
+
R'
R'
R
R
R'
R'
1)FeCl
3
85%
2)benzene/oleum 95
%
Δ
21%
O
O
R'
R'
R'
R'
R
R
R,R'= alkyl chains
R
R
41
42
R
R
40
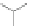
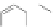
Scheme 12 Synthesis of supernaphthalene 42 [
34
]
O
1) 180-200°C, 80%
2)TBAF, quant.
+
43
TIPS
TIPS
44
45
tetraphenylcyclo-
pentadienone, reflux
80%
CuCl
2
/AlCl
3
, 100°C
47
46
Scheme 13 Iterative Diels-Alder/deprotection strategy for the synthesis of nano-graphene
47 [
35
]
Interestingly, even oligophenylenes with overlapping phenyl groups have been
converted to planar graphene-type structures upon cyclodehydrogenation through a
skeletal rearrangement [
37
,
38
]. By changing the
meta
connection at the central
benzene ring to
the supernaphthalene 50 was synthesized (Scheme
14
).
Coronene 16 can be viewed as a “superbenzene.” The series was continued with
the synthesis of supernaphthalene 53 and supertriphenylene 56, using different
phenylacetylenes and tetraphenyl substituted cyclopentadienone. Two selected
examples 53 and 56 are shown in Scheme
15
[
39
,
40
].
para